Study of Heavy Metals (Lead and Magnesium) Stress on Rosa indica
Iqra Azam1, Bakhtawar Sajjad1, Hina Sarwar1, Aleeza Javeed1, Anam Riaz1, Waheed Yaseen1, Humayun Ajaz1
1: Universty of Engineering and Technology, Lahore, Pakistan
Corresponding Author: Bakhtawar Sajjad Email: bakhtawarsajjad898@yahoo.com Citation | Azam I., Sajjad B., Sarwar H., Javeed A., Riaz A., Yaseen W., Ajaz H.” Study of Heavy Metals (Lead and Magnesium) Stress on Rosa indica”. International Journal of Agriculture and Sustainable Development, Vol 01 Issue 04: pp 115-138, 2019. DOI | https://doi.org/10.33411/IJASD/2019010409 Received | Nov 25, 2019; Revised |Dec 10, 2019 Accepted | Dec 15, 2019; Published | Dec 27, 2019.
___________________________________________________________________________
Abstract
In the present study, concentration effects of different heavy metals (Pb and Mg) in Rosa indica are observed to detect the changes in growth response due to effect of metal’s toxicity. Both metals have different tolerance index. Lead is toxic metal and has small tolerance index, whereas Mg is a macronutrient having a high tolerance index which can move in a large scale to different parts of the plants. The least and highest accumulation values of Pb and Mg were observed as 0.608-25.897 mg/L and 20948-52291 mg/L, respectively. The toxicity order in rose plant is Pb>Mg. The bio concentration and translocation factor values of Pb and Mg were 0.97, 0.77, 0.93 and 0.9 respectively. In lead polluted soil, plants height shown is declined due to the concentration of lead (66.38 cm-56.48 cm) whereas plant height increased because of massive concentrations of Mg (63.93 cm-75.03 cm). Results `revealed that accumulation of both metals were excessive in the roots and least in the stem and leaves. It is concluded that rose plant is a good accumulator of lead and magnesium.
Keywords: Plant height, toxicity order, heavy metals, bio-concentration factor, translocation factor, Rosa indica
Introduction.
Pollution of heavy metals in soil is constantly increasing which is causing the soil tarnishing. This situation is alarming and creating worldwide concern. Due to heavy metals accumulation in soil large areas have been changed into toxic hotspots. It is creating bad impact on ecosystem [1] Heavy metals comprises almost thirty-eight (38) elements. Although some of them are essential for the growth of plants [2] yet heavy metals are phototoxic substances and accumulating major environmental pollutants. Due to accretion of automobiles and population density, serious environmental risks have taken place. The metal level in soil ranges from less than 1 to 100000 mg kg-1. Metals are being inducted in soil by different sources from many years which causes negative impact on micro flora, food chain, quality of drinking water, plant growth, human health and dwindle of crop production [3]. Anthropogenic activities have increased the level of metals in the environment and cause a serious problem for human beings [4]. Although small number of heavy metals are necessary for human life as well as every creature, but ultra toxic level causes serious problems [5]. The exposure of industrial waste for production of crops and other products increase the level of heavy metals[6]. Heavy metals polluted soil consists of convertible ions, absorbed inorganic solids, non-exchangeable ions, insoluble metal compounds such as carbonates and phosphates. Metal Stress on Plant Growth:
The excessive level of heavy metals have bad effects on plant growth directly and indirectly. Direct effects involve suppression of cytoplasmic enzymes and cell death due to metal stress. Indirect effects include substitution of nutrients at cation exchange points in plants [7].Heavy metals stress has detrimental effects on the metabolic activities of plants, and further DNA reproduction, leaf morphology, chromosomal pulverization, chlorophyll aggregation, biosynthesis and cell division. Toxic level of heavy metals causes to truncate photosynthetic pigments . [8]. It also generate the oxidative stress by damaging the metabolic series. This results in variation of the production of reactive oxygen species [9].
Heavy metals cannot be biologically demolished but can be converted from one oxidation state to others [10].
Heavy metals generate the phytotoxicity which results in retardation of plant growth, distortion of enzymes function, reduction of plant biomass, cell death, ion leakage, deterioration of RNA, vein necrosis, loss of turgor pressure, deterioration of protein content, leaf chlorosis and reduction of leaves and roots growth [11].
Uptake of Heavy Metals.
Plants need macronutrients (N, S, K, P, Ca and Mg) as well as essential micronutrients (Fe, Ni, Mn, Cu and Mo) for growth and completion of life cycle. Essential metals (Mn, Cu, Zn, Fe, Mg, Co, Se, Cr, Mo, and Ni) are used to generate physical activities such as cofactor in oxidation-reduction reactions. Non-essential metals (Cd, As, Pb, Hg, Sb, and Ag) disrupt the enzymatic reactions due to their interaction with thiol groups [12].
In plants, metals can be transmitted and aggregated by use of metal transporters [13]. Plants have special mechanisms to take up, transfer and store nutrients. Due to oxidative destruction, contamination metals enhances in plants but the antioxidants presence conserve the plants from oxidative loss. These antioxidants manage the generation of reactive oxygen species [14].
Metal bioavailability depends upon physiochemical properties pH of the soil, cation exchange capacity, organic matter and the solubility of the metal in soil. In acidic soil, the dissociation of metal from soil activates due to the struggle of the proton for binding sites. In the soil solution bioavailability of metals can be determined by factors such as quality of soil, atmospheric conditions, root system and plant’s genotype [15].
Plant’s Resistance against Absorption of Heavy Metals:
Several plants can aggregate heavy metals while few of them eliminate them. Different parts of the plants exhibit the different tolerance index towards heavy metal accumulation.
Plants growing in the metal polluted soil are classified as accumulators, excluders and indicators [161]. Accumulators absorb an excessive amount of pollutants into aerial tissues. They convert pollutants into the inert form and allow accumulation of a large amount of metal. From 45 different plant families, 400 plants have been reported as accumulators of metals. These plants accumulate greater than 10 ppm Hg, 100 ppm Cd, 1000 ppm Co, Cr, Cu and 10000 ppm Ni and Zn. Excluders impede to uptake of pollutants into their biomass. Indicators have poor control on uptake of metal and further transmistting processes [17]. Heavy metals may not reserved in the roots. Plants transmit the metals towards shoots and assemble in leaves. The practice of concentration of metals has been seen in 100-1000 folds which are higher than observed in non-hyperaccumulators. Over 450 angiosperm species are recognized as accumulators of heavy metals [18]. When the level of reactive oxygen species increases then defensive enzymes such as catalase (CAT), superoxide dismutase (SOD), and peroxidase (POD) accommodated in plant cells serve as scavengers [19].
Abatement of Environmental Pollution:
Soil contaminated, due to heavy metals is hard to remediate. Environmental pollution can be reduced by using of different ways. The economical method is to use the plant species for removal of metals from contaminated soil. Reduction of environmental pollution by plants is an effective and economical method. Elimination of heavy metals from the soil through lime congealing, ion exchange, electroplating, solvent extraction and chemical precipitation are expensive methods and have many drawbacks such as the formation of poisonous wastes, partial removal of metal, for which high energy is needed.
At the high metal concentration plants proceed in two ways. First is the evasion mechanism in which plants inhibit retention and transport metals into their tissues. These are known as non-accumulators. Second is the metal accession mechanism in which plants exhibit massive potential for uptake of metals by roots and transport them towards the stem. These are known as hyper accumulators. The mechanism of hyperaccumulation of metals by plants depends upon three features:
a. plants ability to uptake of heavy metals from soil,
b. rapid and efficient transfer of metals from roots to shoots,
c. plant potential to detoxify, segregate and further transmission plenty amount of metals in the leaves.
Choice of Plant.
In this study, we have assessed the level of heavy metals such as lead (Pb) and magnesium (Mg) in stem, leaf and roots of Rosa indica. Selected soil was contaminated due to accumulation of heavy metals (Pb and Mg) which have toxic effects on plant growth, as well as animals and food chain. The objective of this study is to measure the growth of plants (Rosa indica) in response of toxicity level and tolerance index of heavy metals (Pb and Mg) in Rosa. Interaction of metals with plants has two aspects. One is the adverse effect of heavy metals on plants which results in growth inhibition and second one is the ability of some plants to detoxify effects of heavy matels contamination by their own defence system. Rosa indica belongs to the family of Rosaceae. It is perpetual flowering shrub of genus Rosa. Rosaceae is the 19th largest family contains more than hundred genera which are worldwide grown. Chinese were the first who grown roses 2000 years ago. Demand of roses has been raised at higher rate with contrast to other flowers [20].
Rose is part of nature attractive creation and universally known as the Queen of flowers due to its brilliant color, elegant fragrance, charming shape and varying sizes. Globally 150 species of roses are found, out of which 34 species are cultivated in India. There exist more than 20,000 economical species of rose. Roses are cultivated by using seeds, trimming, grafting and layering. Nowadays roses are grown by using tissue culture technique. Roses are mostly used at social occasions as well as religious ceremonies [21].
Essential oil of rose contains aliphatic mono-terpene alcohols, nerol (20%), geraniol (75%), phenyl ethanol and citronellol (20%) [22]. Nonadecane, pentacosane, tricosane and heneicosane are aliphatic hydrocarbons of rose water and essential oil. Roses have plenty of vitamin C which is used for preparation of certain medicines.
In Unani medicines rose has vital importance. It is described as dermatitis, cardiotonic and expectorant. Essential oil from rose petals is used to prepare perfume, rose oil, gulkand and gulroghan. Rose oil has positive impact on digestive tract disorder and also used to remediate skin problems.
The flowers of rose are used to handle unpleasant odor from mouth, to control high blood pressure, curb high cholesterol, treatment of leprosy and burning sensation due to its antipyretic and aphrodisiac activity. Floral extract of plant has antifungal activity while ethanolic extract possessed antibacterial exertion against pathogens. Buds and petals of Rosa indica are used for removal of stones in kidney and gall bladder. It is also used to cure chest problems, sore throat, eye diseases, bacterial infections, blocked bronchial tubes, asthma and runny nose [23].
Roses are also used for clinical purposes and comprise of coloured stains and chemical substances like flavonoids. The tea produced from the petals and leaves of the rose plant used to minimize the cold and pyrexia. It is also used as a diuretic which eliminate the excess of toxic material from the body.
Rose hips have dietary importance and used in cooking for food flavoring and also give beautiful colors to food. The rose plant plays important role in antidepression, antioxidant and anticarcinogenic activities.
Material and methods:
Plantation.
The experimental work was conducted by purchasing the rose seedlings. The seedlings and organic soil were purchased from Malik Nursery Sagian Wala Bypass, Service Road Al Hamad Colony Lahore. The seedlings were transferred into seven different pots of equal length and filled with organic soil. The plants were watered for one week then the metal stress was applied for four weeks.
Sampling.
All the selected seedlings were of uniform size. Every pot was 6 inches wide from the upper side, 3 inches wide from the lower side and 6 inches long. All the pots were labelled properly. pH of the soil was 7.56. Metal stress was applied from 6th May to 3rd June 2018 at an average temperature of 30℃. 1000 ml solution of different strengths (50 mg/L, 100 mg/L and 150 mg/L) of Pb and Mg were applied every day to individual pots for a total period of 28 days (1st to 4th weeks). In addition, control experiment was also conducted. The metal uptake was estimated once in every week.
Collection of Samples:
Leaves, stems and roots from each replicate were cut down after every week. The samples were first washed under a stream of water and then with distilled water. The samples then dried under sunlight for one day and stored in polyethene bags.
Sample Analysis.
The samples were analyzed by using the following steps.
Heating of samples by using an electric oven:
All the samples were heated for four to five hours at 120℃.
Crushing of Samples:
After heating in the oven the samples were grinded into the fine powder and used for metal analysis.
Sample weighing:
0.1 g crushed sample of leaves, stem and roots was weighed by using weighing balance.
Digestion of plant sample:
The weighing samples (0.1 g) were digested with aqua regia for extraction of metals. Aqua regia is the mixture of concentrated nitric acid and hydrochloric acid in the ratio of 1:3 respectively. It is the most effective method for metal analysis. 10 ml of freshly prepared aqua regia was added in each sample and left for two days. All the beakers were covered with the glass plate.
Dilution of the sample:
40 ml of distilled water was added in each digested sample to make the volume up to 50 ml.
Filtration of sample:
The diluted sample was filtered with Whatman filter paper.
Storage of sample:
The filtrated samples were stored in the 150 ml bottles by properly lading and labelling.
The sample analysis (Pb, Mg) was run in variance flame atomic absorption spectrophotometer.
Result and discussion:
Effect of Lead:
The rose plant has shown variations toward the different concentrations of heavy metals (Pb and Mg). Absorption of lead was estimated in roots, stem and leaves of rose plant and obtained results were statistically analyzed (T test and Q test). In controlled experiment at 0 mg/L the absorption values in roots, stem and leaves were 0.177, 0.111 and 0.017 respectively. The lead accumulation was observed as 50 mg/L in roots, stem and leaves were 1.139, 0.876 and 0.775 respectively. The absorption of Pb in 100 mg/L was found as 2.239, 1.946 and 1.543 in roots stems and leaves. The absorbed amount in 150 mg/L lead in roots, stem and leaves were 3.294, 2.568 and 2.068 respectively.
The total amount of lead accumulated in roots, stem and leaves at 0 mg/L Pb, 50 mg/L Pb, 100 mg/L Pb and 150 mg/L Pb were 0.305, 2.79, 5.728 and 7.93 respectively.
Table 1: Absorbance and different concentration of lead in roots, stem and leaves of Rose plant.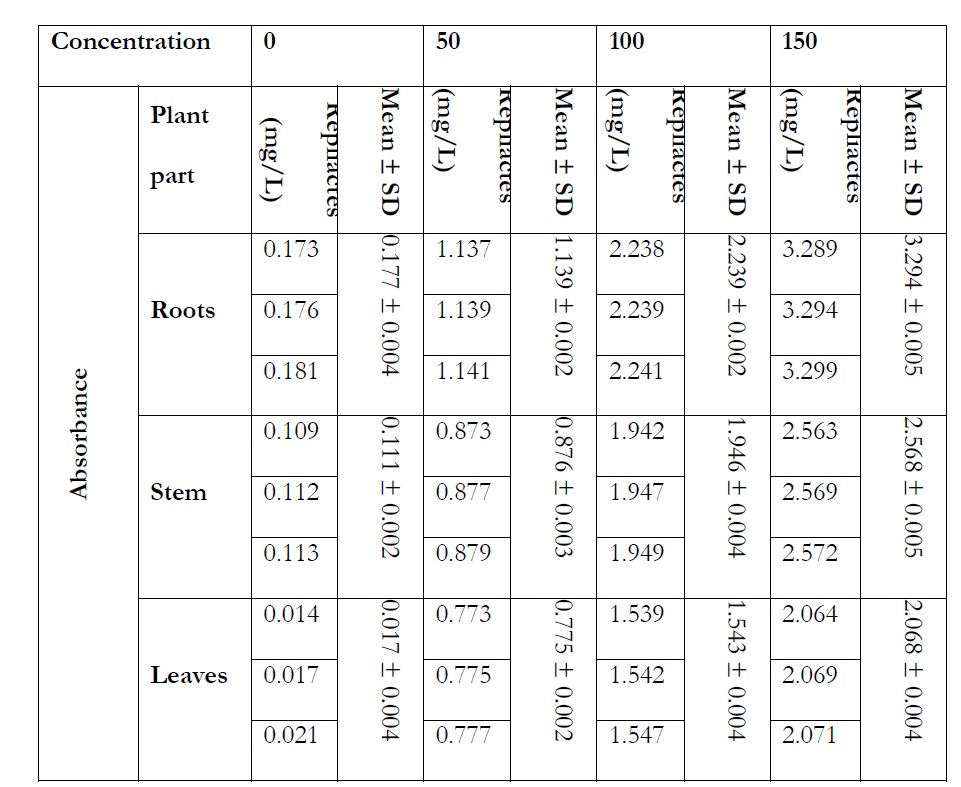
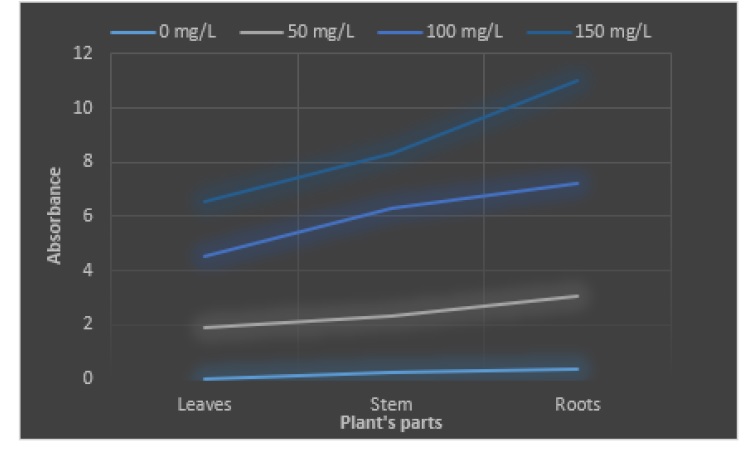
Figure 1 Comparative relation between absorbance of Pb in roots, stem and leaves
by increasing the concentration of lead and number of day’s plant’s height was greatly affected.
Table2: Plant’s height on different concentrations of Pb and Mg.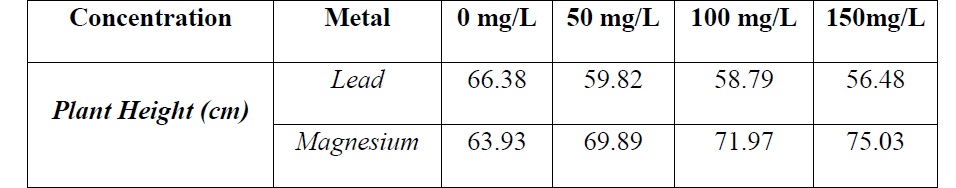
Similar result was found in research paper [24] studied for the effect of different concentration of Pb on spinach and wheat. Lead proceeded to all parts of plant (having low tolerance index) and absorption rate from roots to leaves increased linearly with increasing concentration of lead. The amount of lead ranging from 100-200 ppm caused chlorosis, deficiency of essential nutrients, tip burn, leaves yellowing and lessen the plant growth.
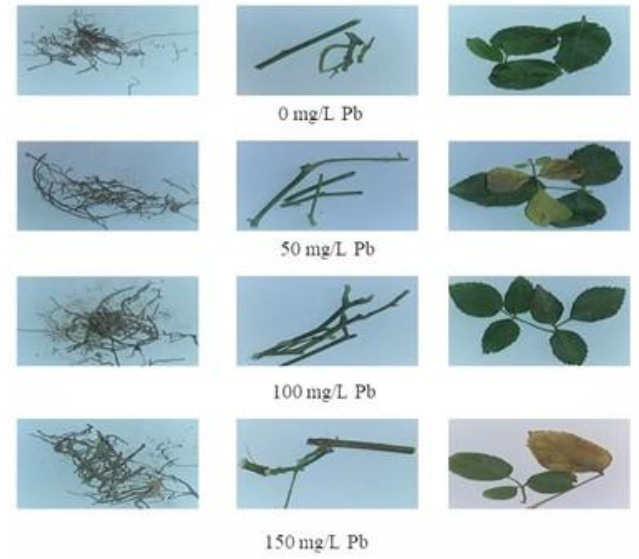
Figure 2 Effect of Pb on Rose Plant
The toxic order of Lead accumulation in different parts of plant are as follows:
Leaves ˂ Stem ˂ Roots
Effect of Macronutrient Magnesium:
Magnesium is a macronutrient for rose plant. The rose plant shows variations towards different concentrations of heavy metals (Pb and Mg). Absorption of magnesium was estimated in roots, stem and leaves of rose plant and obtained results are statistically analyzed (T test and Q test). In controlled experiment at 0 mg/L the absorption values in roots, stem and leaves were 2635, 2317 and 1818 respectively. The accumulation of magnesium at 50 mg/L in roots, stem and leaves were 3651, 3130 and 2927 respectively. The absorption at 100 mg/L Mg in roots, stem and leaves were 4447, 3995 and 3632 respectively. The absorbed amount in 150 mg/L magnesium in roots, stem and leaves were 5485, 4978 and 4658 respectively. The total amount of magnesium accumulated in roots, stem and leaves at 0 mg/L Mg, 50 mg/L Mg, 100 mg/L Mg and 150 mg/L Mg were 6770, 9708, 12074 and 15121 respectively
Table 3. Absorbance of different concentration of magnesium in roots, stem and leaves of Rose plant.
Concentration
Figure 3 Comparative relation between absorbance of Mg in roots, stem and leaves.
Plant’s height increases gradually by increasing the concentration of magnesium in a number of days. It was observed that concentration of magnesium above 150 mg/L extended the plant growth. Magnesium travelled to all parts of plant (due to high tolerance index) and absorption rate increased linearly with intensifying concentration of magnesium. Magnesium deficiency caused yellowing of leaves, leaf senescence and suppresses the plant growth and yield.

Figure 4 Effect of Mg on Rose plant
The accumulation of magnesium in different parts of plant is as follows:
Leaves ˂ Stem ˂ Roots
Statistical analysis:
For statistical analysis of metal accumulation, two tests are used one is test method (Student T test) and other is Q test prosecuted to observe the rejections in obtained results.
Hₒ or Null hypothesis:
If value t ˃tv then the calculated value of metal absorption by plant, the flaw in the experiment is not happened by chance. Error is determined and occur due to issues in working of instrument AAS e.g., metal verification, instrumental zero, and lamp hindrance. The error may also be possible due to imprecise preparation of solutions, flaws in acid digestion and crushing and weighing of samples.
H1 hypothesis:
The experimental error takes place by chance if t ˂ tv. The indeterminate error can be present but not large enough to reject H1 hypothesis.H1 hypothesis may or may not be rejected if t ˃ tv but the value is regarded as suspicious value. The desertion of suspected value can be tested by Q test. If value is Q ˂ Q95 or t˃ tv then test is not rejected and error in the experiment may be indeterminate.
Student’s T and Q test for Pb.
The calculated T test values in 0 mg/L Pb roots and stem were 4.347 and 4.478 respectively. Similarly, the values for 50 mg/L Pb in stem and 100 mg/L Pb was in leaves 4.381 and 4.347. All these t values are greater than tabulated value (tv) at confidence limit 95%. The 95% probability revealed that the error is determinant and not occurred by chance.
Table 4. Student T test statistical analysis of different parts of Rose plant against different concentration of Pb.

The suspected values in each case can be tested by Q test. The calculated Q values at 0 mg/L Pb roots and stem were 0.62 and 0.25. While the Q values for 50 mg/L Pb stem and 100 mg/L Pb in leaves were 0.33 and 0.62 respectively. The suspected values were less than the tabulated value (Q ˂ Q95). Consequently 95% probability was that, suspected values were not outlier of the range, so results were not rejected.
Table 5.Q test Rejection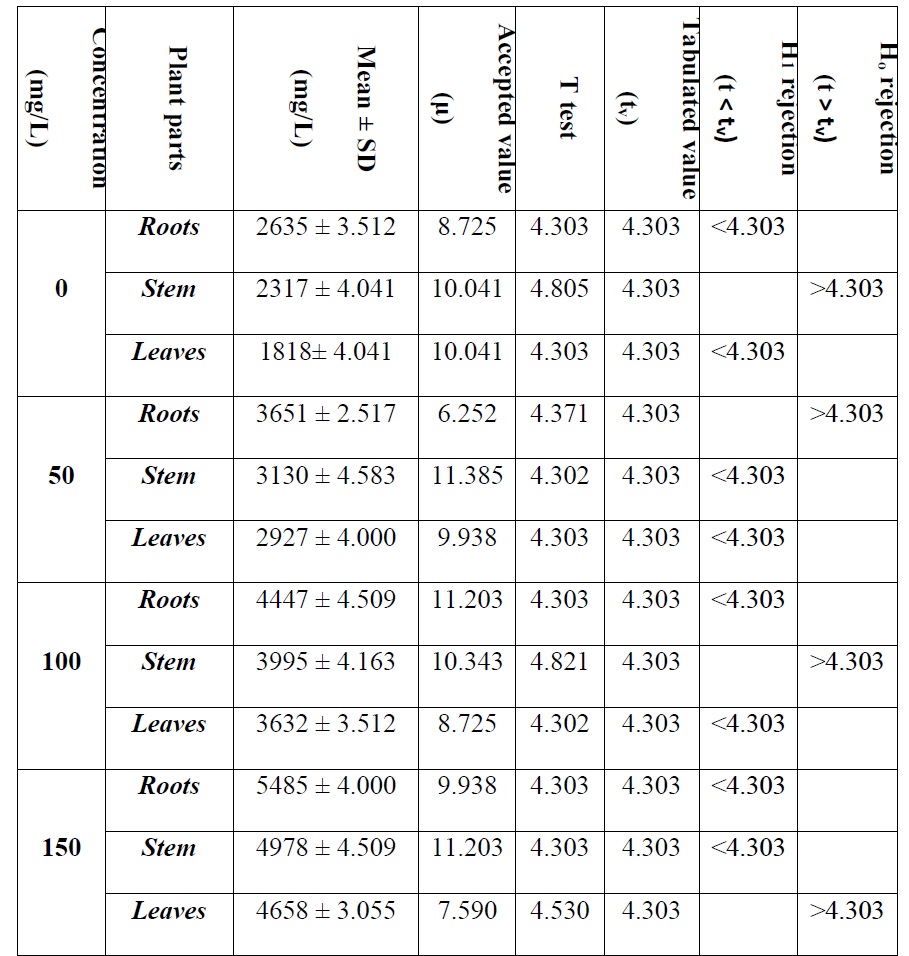
Student T test and Q test for Mg.
The calculated T test values in 0 mg/L Mg stem was 4.805. Similarly, the values for 50 mg/L Mg in roots, 100 mg/L Mg in stem and 150 mg/L Mg in leaves were 4.371, 4.821 and 4.530 respectively. All these t values are greater than tabulated value (tv) at confidence limit 95%.
Table 6. Student T test statistical analysis of different parts of Rose plant against different concentration of Mg.

The calculated Q value at 0 mg/L Mg stem was 0.62. While the Q values for 50 mg/L Mg roots, 100 mg/L Mg in stem and 150 mg/L Mg in leaves were 0.4, 0.25 and 0.66 respectively.
The suspected values were less than the tabulated value (Q ˂ Q95) consequently 95% probability that suspected values were not outlier of the ranges, so results were not rejected. 95% confidence was that, error in the experiments were not due to determinate so it was quite possible that error may be occurred by chance or indeterminate.
Table 7.Q test
Rejection test of suspected value of different parts of Rose plant at the different concentration of Mg.
Translocation and Bioconcentration Factor.
The metal transfer rate from the roots to shoots depends upon level of metals in roots. The migration of heavy metals from polluted soils towards plant roots and its potential to transfer metal from roots to aerial parts of plant were estimated by means of bioconcentration factor and translocation factor.
The translocation factor of metals in different parts of Rosa indica was calculated by using the following formula:
???????????????????????????????????????????????????? ????????????????????????= ???????????????????????????????????????????????????? ???????? ???????????????????? ???????? ???????????????????????????????????????????????????????????????????? ???????? ???????????????????? ???????? ????????????????????Factor= Concentration of metal in stem/concentration of metal in roots
Bioconcentration factor used to calculate plant capability to accumulate heavy metals from soil.
???????????????????????????????????????????????????????????????? ????????????????????????=???????????????????????????????????????????????????? ???????? ???????????????????? ???????? ???????????????????????????????????????????????????????????????????????? ???????? ???????????????????? ???????? ????????????????on Factor=Concentration of metal in roots/Concentration of metal in soil
Discussion.
In the present study, plant physiology was investigated under the toxic effects of different concentrations of Pb and Mg in soil. The plant growth and biomass increase was effected with the concentration of massive quantity of magnesium, which is due to the reduction of oxidative stress, increase in antioxidant enzyme activity, while plant growth decreases with increasing concentration of heavy metal lead. The capacity of plant to absorb heavy metals is an effective remedial measure for treatment of metal contaminated soil.
The accumulation of heavy metals in plant’s parts is exhibited in Roots > Stem > Leaves while the order of toxicity is Pb> Mg. The distribution of toxicity factor and tolerance of heavy metals in rose plant is Mg >Pb. Results reveals that Rosa indica has potential to tolerate and accumulate both Pb and Mg elements.
Conclusion
The massive quantity of lead and magnesium accumulated in soil travel from roots to leaves. Four weeks samples disclosed accumulation of maximum amounts of both Pb and Mg. Concentration of lead results in decline of plant growth on the other hands increase of magnesium level above to 150 mg/L up lift the plant growth. It is observed that rose plant accumulates magnesium in large amount as compared to lead. Based on the bio concentrations and translocation factors values, the plant species is a good accumulator of these two metals. Due to excess level of metals in rose plant, the plant does not show flowering, medicinally effectiveness as well as ornamental uses. For proper growth and beneficial uses rose plant should be planted in unpolluted soil. Finally, it is concluded that the soil contaminated with extraordinary accumulation of both metals may affect on growth of almost all kind of plants.
Anyhow it is also proved that the soil polluted with the both metals at lower stages may treated with the plantation of rose as this method is economical and effective.
Acknowledgement. Iqra Azam, Bakhtawar Sajjad, Hina Sarwar, Aleeza Javeed, Anam Riaz and Waheed Yaseen are sincerely thanks to the University of Engineering and Technology Lahore for conducting this research and thankful to the Humayun Ajaz for his guidance and assistance throughout the research.
Author’s Contribution. All the authors contributed equally.
Conflict of interest. We declare no conflict of interest for publishing this manuscript in IJASD.
Project details. NIL
References.
1. V. C. Pandey, D. N. Pandey, N. Singh, “Sustainable phytoremediation based on naturally colonizing and economically valuable plants,” Journal of Cleaner Production, vol. 30, pp. 1–3, 2014.
2. S. P. Tripathi, U. P. Singh, B. K. Pandey, “Detrimental Effects of Selected Salts on Seed Germination and Growth Parameters of Spinach (Spinaciaoleracea),” International journal of Current Microbiology and Applied Sciences, vol. 6, no. 9, pp. 2213–2217, 2017.
3. Esringu, E. A. Kulekci, M. Turan, S. Ercisli, “Phytoremediation of Some Heavy Metals By Different Tissues of Roses Grown in The Main Intersections in Erzurum City, Turkey,” Journal of Fresenius Environmental Bulletin, vol. 24, no. 9, pp. 2787–2791, 2015.
4. V. Subhashini, A. V. V. S. Swamy, “Phytoremediation of Heavy Metals Contaminated Soils by Catharanthusroseus,” International journal of Science and Research, vol. 5, pp. 726–729, 2016.
5. A. Aman, D. Ahmed, N. Asad, R. Masih, H. M. Rahman, “Rose biomass as a potential biosorbent to remove chromium, mercury and zinc from contaminated waters,” International journal of Environmental Studies, vol. 10, pp. 1–14, 2018.
6. N. Ehsan, R. Nawaz, S. Ahmad, M. M. Khan, J. Hayat, “Phytoremediation of Chromium contaminated soil by an Ornamental plant (Vincarosea),” Journal of Environmental and Agricultural Sciences, vol. 7, pp. 29–34, 2016.
7. A. Asati, M. Pichhode, K. Nikhil, “Effect of Heavy Metals on Plants,” International Journal of Application or Innovation in Engineering and Management, vol. 5, pp. 56–66, 2016.
8. W. Wani, K. Z. Masoodi, A. Zaid, S. H. Wani, F. Shah, V. S. Meena, S. A. Wani, K. A. Mosa, “Engineering plants for heavy metal stress tolerance,” Journal of RendicontiLincei. ScienzeFisiche e Naturali, vol. 10, pp. 122–137, 2018.
9. M. Jaskulak, A. Grobelak, “Cellular mechanisms of heavy metals accumulation, detoxification and tolerance in hyperaccumulating plants,” Journal of Contemporary Problems of Power Engineering and Environmental Protection, vol. 11, pp. 127–132, 2017.
10. B. Kumar, K. Smita, L. C. Flores, “Plant mediated detoxification of mercury and lead,” Arabian Journal of Chemistry, vol. 10, pp. 2335–2342, 2017.
11. D. Kapoor, S. Kaur, R. Bhardwaj, “Physiological and Biochemical Changes in Brassica juncea Plants under Cd Induced Stress,” International Journal of BioMed Research, vol. 2014, pp. 1–13, 2014.
12. S. Dubey, M Shri, A. Gupta, V. Rani, D. Chakrabarty, “Toxicity and detoxification of heavy metals during plant growth and metabolism,” Journal of Environmental Chemistry Letters, vol. 8, pp. 101–124, 2018.
13. M. P. Mourato, I. N. Moreira, I. Leitao, F. R. Pinto, J. R. Sales, L. L. Martins, “Effect of Heavy Metals in Plants of the Genus Brassica,” International Journal of Molecular Sciences, vol. 16, pp. 17975–17998, 2015.
14. L. Du, X. Xia, X. Lan, M. Liu, L. Zhao, P. Zhang, Y. Wu, “Influence of Arsenic stress on Physiological, Biochemical, and Morphological Characteristics in Seedlings of Two Cultivars of Maize (Zea mays),” Journal of Water Air Soil Pollution, vol. 55, pp. 1–14, 2017.
15. M. M. Lasat, “The Use of Plants for the Removal of Toxic Metals from Contaminated Soil,” Journal of Environmental Science and Engineering Fellow, vol. 2, pp. 1–33, 2015.
16. S. Ramana, A. K. Biswas, A. B. Singh, N. K. Ahirwar, A. S. Rao, “Potential of Rose for phytostabilization of chromium contaminated soils,” Indian Journal of Plant Physiology, vol. 18, no. 4, pp. 381–383, 2013.
17. V. Subhashini, A. V. V. S. Swamy, “Phytoremediation of Pb and Ni Contaminated Soils Using Catharanthusroseus,” Universal Journal of Environmental Research and Technology, vol. 3, pp. 465–472, 2013.
18. N. Rascio, F. N. Izzo, “Heavy metal hyperaccumulating plants: How and why do they do it? And what makes them so interesting?,” Journal of Plant Science, vol. 180, pp. 169–181, 2011.
19. A. Emamverdian, Y. Ding, F. Mokhberdoran, Y. Xie, “Antioxidant Response of Bamboo (IndocalamusLatifolius) as Affected by Heavy Metal Stress,” Journal of Elementology, vol. 23, pp. 341–352, 2018.
20. J. R. Chavada, B. V. Thumar, A. N. Vihol, V. S. Petal, B. M. Padhiyar, “Effect of Potting Media on Growth, Flower Yield, and Quality of Rose (Rosa hybrida) Top Secret under Protected Condition,” International
21. V. S. Patel, V. R. Malam, K. H. Nurbhanej, A. N. Vihol, J. R. Chavada, “Effect of organic manures and biofertilizers on growth flowering and flower yield of rose (Rosa hybrida),” International Journal of Chemical Studies, vol. 5, pp. 1924–1927, 2017.
22. H. M. Rsheed, T. Khan, F. Wahid, R. Khan, A. J. Shah, “Chemical Composition and Vasorelaxant and Antispasmodic Effects of Essential Oil from Rosa indicaL. Petals,” Journal of Evidence Based Complementary and Alternative Medicine, vol. 2015, pp. 1–9, 2015.
23. S. Bai, L. Seasotiya, A. Malik, P. Bharti, S. Dalal, “Bioactive compounds and pharmacological potential of Rosa indicaL. and PsidiumguajavaL. methanol extracts as antiurease and anticollagenase agents,” Journal of Der Pharmacia Lettre, vol. 7, pp. 179–184, 2015.
24. M. Lamhamdi, O. E. I. Galiou, A. Bakrim, “Effect of lead stress on mineral content and growth of wheat (Triticum aestivum) and spinach (Spinacia oleracea) seedlings,”Saudi J. Biol Sci, vol. 20, pp. 29-36, 2013.



