Detection of Coronary Artery Using Novel Optimized Grid Search-based MLP
Iftikhar Hussain1, Raja Rizwan Javed2, Huma Qayyum1, Farman Hassan1, Auliya Ur Rahman1
1 University of Engineering and Technology Taxila, Punjab Pakistan
2 National Defense University, Islamabad
* Correspondence: Farman Hassan, Email ID: farmanhassan555@gmail.com.
For Citation | Hussain. I, Javed. R.R, Qayyum. H., Farman. H, Rahman. A, “Detection of Coronary Artery Using Novel Optimized Grid Search-based MLP”. International Journal of Innovations in Science and Technology. Vol 4, issue 1, 2022, pp: 276-286.
Received |Jan 11, 2022; Revised | Feb 15, 2022 Accepted |Feb 26, 2022; Published | March 16, 2022 ___________________________________________________________
In recent years, we have witnessed a rapid rise in the mortality rate of people of every age due to cardiac diseases. The diagnosis of heart disease has become a challenging task in present medical research, and it depends upon the history of patients. Rapid advancements in the field of deep learning. Therefore, it is a need to develop an automated system that assists medical experts in their decision-making process. In this work, we proposed a novel optimized grid search-based multi-layer perceptron method to effectively detect heart disease patients earlier and accurately. We evaluated the performance of our method on a dataset named Public Health dataset for heart diseases. More specifically, our method obtained an accuracy of 95.12%, precision of 95.32%, recall of 95.32%, and F1-score of 95.32%. We made a comparison of our method with existing methods to check superiority and robustness of our system to detect heart disease patients. Experimental results along with comprehensive comparison with other methods illustrate that our technique has superior performance and is robust to detect heart disease patients. From the results, we can conclude that our method is reliable to be used in hospitals for the early detection of heart disease patients.
Keywords: heart disease, coronary artery narrowness, block vessels, heart attack, deep learning, intelligent systems.
|
Author’s Contribution. Iftekhar Hussain, Huma and Auliya designed the overall working plan of this manuscript, Raja Rizwan Javed worked on various models e.g., ICA-SVM, AWAIS, KNN and N2Genetic-NuSVM to examine the percentage of |
accuracy of these models to detect cardiac diseases. Farman Hassan thoroughly read this manuscript and added considerable corrections to device the novel technique. Acknowledgment. We acknowledge the UET Taxila for |
providing us all resources to complete this work. Conflict of interest. Authors claim that there exists no conflict of interest for publishing this manuscript in IJIST.
|
INTRODUCTION
Cardiac disease describes numerous heart-related conditions, which include various heart diseases such as arrhythmias, congenital heart defects, coronary artery disease, and narrowness of blood vessels. Sometimes cardiovascular disease term is used interchangeably for heart disease. CD is mostly referring to the conditions in which the blood vessels are blocked or narrowed that may cause myocardial infarction (stroke or chest pain). Other abnormal conditions such as those affecting rhythm of the heart, valves, or muscle are also considered cardiac diseases. Recently, the health sector collected a large amount of patients’ history and diagnoses of different diseases. However, practitioners and the research community don’t use the data effectively. Providing quality service is a challenging and crucial task for healthcare centers. The quality of service includes providing effective treatment and accurate diagnosis of diseases. Poor diagnosis of diseases can lead to serious consequences and sometimes it takes the life of a person. Cardiac disease has numerous risk factors such as ethnicity, family history, increasing age, and male hood. Every year, millions of people die of heart attacks, and a total of 31% of the deaths in the world occurred due to heart attacks. In present times, cardiac disease is the most leading impact of fatality around the world. Therefore, health sectors require to enhance the prediction of cardiac diseases [1] through deep learning methods. Accurate diagnosis of cardiac disease predominantly depends on the previous records of patients [2]. Hence, risk factors that cause cardiac diseases such as diabetes, abnormal blood pressure, chest pain, cholesterol, cigarette smoking, and sex [3-5] need to monitor all the time. Researchers use these risk factors to design intelligent systems to detect cardiac diseases earlier.
The research community has explored various ECG-based and data mining-based approaches [6-20] to detect cardiac diseases. Deep learning has shown a noticeable enhancement in the detection and analysis of cardiac disease [7]. In [8], a convolutional neural network (CNN) was used to detect cardiac disease using an electrocardiogram (ECG). In [9], a cardiac disease diagnosis system was designed based on electrocardiography. In [10], the deep neural network was employed to diagnose cardiotocographic of fatal assessment that was based on multiclass morphologic patterns. This has been analyzed that cardiac disease is a term used for many types such as coronary, congenital heart disease, and rheumatic. Therefore, heart activity was analyzed during exercise times, working, and while in rest [11,12]. There are various risk factors of cardiac diseases such as sweatiness, respiratory shortness, discomfort, chest pain, heart palpitation, fatigue, and dizziness. In [13], a deep belief network was employed to detect cardiac disease using clinical data and an accuracy of 91.26% was obtained. Similarly, in [14], a deep learning-based approach was designed to detect cardiac disease using ECG data. Few works [15,16] have explored the severity of cardiac disease in patients and employed cardiac magnet resonance imaging, ECG, stress testing, coronary angiogram, chest x-rays to detect cardiac diseases. Data mining and medical science have also been explored to detect various signal types of metabolic syndromes when a person is doing some physical activity such as working, resting, and doing exercise [17]. In [18], heart risk prediction and medicine recommendation systems were explored using big data analytics. In [19], a two-layer gradient boosting decision tree was employed to detect a cardiac disease from the blood data. An accuracy of 86% was obtained for the detection of cardiac disease. In [20], deep learning was employed for cardiac diseases using big data.
Researchers have also investigated various DL-based [21-27] techniques for the prediction of cardiac diseases. In [21], an artificial neural network (ANN) was employed to detect heart diseases. Bioinformatics applications were used to extract patterns from the datasets by employing data mining methods. In [22], an enhanced deep learning assisted convolutional neural network was employed for the diagnosis of heart disease patients. In [23], neural network-based method was introduced for diagnosing of cardio patients. Neural network is comprised of five-level architecture. K-fold cross-validation technique, Matthew’s correlation coefficients, an optimization technique, and a risk-reduction algorithm were also employed to obtain better accuracy. In [24], a deep learning-based approach comprised of Bi-directional long short-term memory and gated recurrent neural network were used to detect heart disease patients. In [25], a healthcare system based on an ensemble deep learning method was designed for the diagnosis of heart disease. Similarly, in [26], various machine learning algorithms such as support vector machine (SVM), logistic regression (LR), k-nearest neighbor (KNN), MLP, and an enhanced recurrent neural network were employed to detect heart disease patients. However, the recurrent neural network performed the best among other techniques. In [27], the neural network ensemble method was designed to diagnose heart disease. The ensemble approach introduced another model that integrated the probabilities of various methods.
The above-mentioned data mining, ECG-based, and deep learning-based approaches have shown significant improvement in terms of accuracy to detect cardiac diseases, however, there still exist limitations such as lower performance of prediction due to employing irrelevant features, redundancy in data, use of features having missing data, worst dimensionality. Therefore, we need an automated and optimized deep learning-based approach that detects cardiac disease patients accurately. In this work, we proposed a novel optimized deep learning approach based on MLP that shows promising classification results to diagnose heart disease patients effectively. The major provisions of this research work are given below,
- This work proposes a unique optimized deep learning approach based on MLP that detects cardiac disease patients accurately and precisely.
- Our system is robust to detect cardiac patients earlier using a minimum number of attributes.
- In order to validate our technique, we conducted extensive experimentation on the Public Health dataset for heart disease patients.
- Experimental results indicate that our technique is trustworthy to be employed for the prediction of heart disease.
PROPOSED METHODOLOGY
There is one main goal of this work, which is to predict the cardiac disease earlier. Our approach comprises four major stages such as pre-processing, optimization, and tuning of hyperparameters for MLP, training, and validation. In the initial stage, we pre-processed the data while in the second stage, we optimized and tuned hyperparameters of MLP. Next, we use the Public Health dataset to train the optimized MLP. Finally, we validated our approach and the system classify the normal person and cardiac disease patient. The flow of our technique is shown in Figure 1.

Figure 1. Proposed System.
Pre-processing
Pre-processing is a crucial step to refine noisy datasets. Missing values and data redundancy decrease the performance of the systems. Therefore, we pre-processed the Public Health dataset for the enhancement of the accuracy of our technique. We checked the dataset for missing values and removed the missing values by zero. The Public Health dataset hasn’t redundant information of cardiac patients.
MLP and Optimization
MLP comprises interconnected nodes or neurons as shown in Fig. 2., which represents a non-linear mapping between input and output vectors. Each neuron is connected by weights and output signals, which are the sums of the input vectors to the node modified by an activation function. Hidden layers of MLP vary from one-to-many hidden layers and a single output layer. MLP is defined as fully connected, with each neuron connected to every other neuron in the forward and previous layer. After configuring a suitable set of weights and activation functions MLP can approximate measurable and smooth functions between an input vector and an output vector. MLP has the capability to learn through training using training data that comprises of a series of input vectors and output vectors. During the training, MLP is frequently presented with weights and training data. Weights are adjusted in-network until the desired mapping of input and output happens. MLP learns using the supervising manner and if mappings do not happen between input and output then an error signal is generated. This error signal is the difference between actual output and desired output. Weights are adjusted during training time according to the magnitude of the error signal to reduce the overall error. MLP can robustly generalize to unseen data, hence, we also employed MLP for the classification purpose of cardiac disease and normal person.
Figure 2. MLP architecture.
Optimization and tuning hyperparameters are the processes of enhancing the performance of a deep learning algorithm by tuning input parameters to fit the model on the data accurately. In deep learning, there is a general concept of loss that shows the poor performance of the model at some specific time interval. We use the concept of loss for training the network to show the performance of the proposed system to detect cardiac disease. Most importantly, we need to minimize the training loss the reason that lower loss during training the model means that the model is performing well. The process of using different input parameters of the model to enhance the prediction performance is called an optimization technique. Optimization techniques are employed to modify the attributes of neural network model and we optimized the performance of the MLP using the following hyperparameters and optimization technique through grid search such as activation function relu, alpha = 0.0001, batch size equal to 16, beta_1 = 0.9, beta_2 = 0.999, epsilon = le-0.8, hidden layer size = 100, learning rate = 0.001, max_fun = 15,000, max iteration = 100, momentum 0.9, solver = adam, validation frequency = 0.1.
EXPERIMENTS AND DISCUSSION
Dataset
In this work, we checked the evaluation of our method (GridSearch-MLP) on the standard public health dataset for cardiac disease [28]. This dataset contains 1025 records of healthy persons and cardiac disease patients. The dataset has 13 attributes and a target variable that has a value of 1 or 0, value 1 represents cardiac disease patients while 0 represents a healthy person. The dataset has 526 records of cardiac disease patients while 499 records of healthy persons. The details of the dataset are available in [28].
Performance of our method GridSearch-MLP
We conducted this experiment for the purpose to check the evaluation of our method optimized neural network grid search based MLP to detect cardiac disease patients earlier. We divided the dataset into two sets such as the training set and the testing set. We utilized 80% of the clinical data to train the optimized MLP while the remaining 20% to test the trained MLP. Comprehensive results of our method is shown in Fig. 3. We examine that our method achieved an accuracy of 95.12%, precision of 95.32%, recall of 95.32%, and F1-score of 95.32%. From the above outcomes, we concluded that GridSearch-MLP has well clustered healthy persons and cardiac disease patients separately. The better precision rate and accurate prediction rate of an optimized MLP indicate that GridSearch-MLP is dependable as well as useful to be used for the detection of cardiac disease. MLP has two properties such as flexibility and adaptive learning, which make the MLP robust for the sequential data while the optimization and tuning hyperparameters enhance the performance to detect cardiac disease. Moreover, we concluded from the results that our method grid search-based optimized MLP is reliable to be used for the detection of cardiac disease.
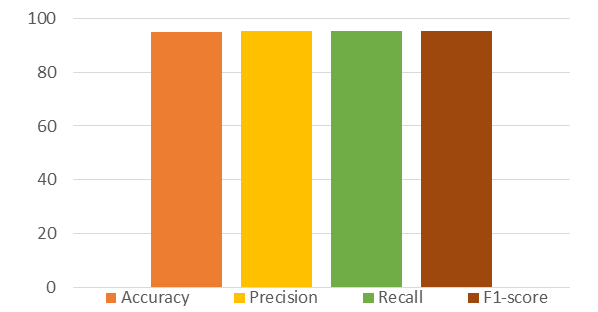
Figure 3. Performance evaluation of the proposed system.
Performance evaluation on machine learning methods
We conducted this experiment for the purpose to compare the performance of the proposed optimized grid search-based MLP against different machine learning algorithms such as logistic regression, decision tree, k-nearest neighbor (KNN), unoptimized MLP, SVM, AdaBoost, gaussian naïve Bayes. From there I, we can observe that KNN performs worst and achieved an accuracy of 74.63%, precision of 75.55%, recall of 72.54%, and F1-score of 74%. Decision tree performs second best and achieved an accuracy of 89.75%, precision of 83.67%, recall of 94.25%, and F1-score of 88.64% while GridSearch-MLP performs best and achieved an accuracy of 95.12%, precision of 95.32%, recall of 95.32%, and F1-score of 95.32%. The detailed results in terms of accuracy, precision, recall, and F1-score are given in Table I.
Table 1: Performance evaluation on machine learning algorithms.
|
Algorithm |
Accuracy% |
Precision% |
Recall% |
F1-score% |
||
|
Logistic regression |
86.34 |
78.57 |
91.66 |
84.61 |
||
|
DT |
89.75 |
83.67 |
94.25 |
88.64 |
||
|
KNN |
74.63 |
75.55 |
72.54 |
74 |
||
|
Unoptimized MLP |
84.87 |
76.53 |
90.36 |
82.87 |
||
|
SVM |
86.82 |
77.55 |
93.82 |
84.91 |
||
|
AdaBoost |
89.26 |
84.69 |
92.22 |
88.29 |
||
|
Gaussian Naive Bayes |
85.36 |
80.61 |
87.77 |
84.04 |
||
|
Grid Search-based MLP |
95.12 |
95.32 |
95.32 |
95.32 |
||
The comparative analysis against the machine learning algorithms, we concluded that the proposed optimized neural network grid search-based MLP is more effective than other traditional methods due to the adaptive learning and flexibility to detect cardiac disease. More specifically, experimental results signify that our method can reliably be used for the detection of heart disease that can assist medical experts in their decision-making process to avoid wrong medication.
Performance comparison with other methods
We conducted this experiment to compare the performance of the proposed system against the existing state-of-the-art methods. For this purpose, we took the experimental results directly from the existing state-of-the-art papers [29-40] without implementing the methods as shown in Table II. The detailed results of the methods [29-40] are given in Table II. From the results reported in Table II, we observed that Sahan et al. [34] performs worst and achieved an accuracy of 82.59%. Abdar, M., et al. [36] employed N2Genetic-NuSVM and achieved an accuracy of 93.08% while the proposed method performs best and achieved an accuracy of 95.12%. Experimental results and comparative analysis of the proposed method against the existing state-of-the-art methods [29-40] illustrate that our method has superior performance to other methods. More specifically, we observe that our method achieved 9.88%, 11.75%, 11.17%, 8.32%, 6.24%, 12.53%, 9.52%, and 2.04% higher accuracy than the existing state-of-the-art methods [29-36]. Comparative analysis signifies the robustness and effectiveness of our method to detect cardiac disease. Hence, we concluded that the proposed method is reliable to be used in health sectors by medical experts for their decision-making process.
Table 2: Performance Comparison with other systems.
|
Author |
Method |
Accuracy% |
|
Helmy, et al. [29] |
Algebraic sigmoid |
85.24 |
|
Wang, et al. [30] |
SVM |
83.37 |
|
Özşen, et al. [31] |
Hybrid similarity measure |
83.95 |
|
Kahramanli, et al. [32] |
Hybrid neural network |
86.8 |
|
Yan. et al. [33] |
ICA-SVM |
88.88 |
|
Sahan et al. [34] |
AWAIS |
82.59 |
|
Duch et al. [35] |
KNN |
85.6 |
|
Abdar, M., et al. [36] |
N2Genetic-NuSVM |
93.08 |
|
Firdaus, F. F., et al. [37] |
DNN-Bayesian Optimization |
91.67 |
|
Qrenawi, Mohammed et al. [38] |
Rmonto ontology-driven data mining approach |
90.0 |
|
Shahid Mehmood et al. [39] |
ANN-based attribute extraction technique |
94.7 |
|
Kaanchan More et al. [40] |
Risk factor-based approach |
86.7 |
|
Proposed |
Grid Search-based MLP |
95.12 |
CONCLUSION
Cardiac disease is deadly, which results in the death of millions of people around the world. In this research, we presented a novel grid search-based MLP to detect cardiac disease earlier. The prediction of cardiac disease is a challenging task as clinical data have redundancy, irrelevant features, and noisy data. Moreover, manual decision-making can lead to serious consequences. Therefore, we proposed an automated system to detect cardiac disease patients earlier based on the previous history of patients. For the experimentation purpose, we used a standard Public Health dataset that is publicly available. We compared the performance of the proposed system with the existing state-of-the-art methods and our method has superior performance. More specifically, we achieved an accuracy of 95.12%, precision of 95.32%, recall of 95.32%, and F1-score of 95.32%. From these results, we concluded that our method is reliable to be used for the detection of cardiac disease.
REFERENCES
[1]. R. Poplin, A. V. Varadarajan, K. Blumer, Y. Liu, M. V. McConnell, G. S. Corrado, L. Peng, and D. R. Webster, ‘‘Prediction of cardiovascular risk factors from retinal fundus photographs via deep learning,’’ Nature Biomed. Eng., vol. 2, no. 3, pp. 158–164, Mar. 2018.
[2]. J. Kim, U. Kang, and Y. Lee, ‘‘Statistics and deep belief network-based cardiovascular risk prediction,’’ Healthcare Inform. Res., vol. 23, no. 3, pp. 169–175, 2017.
[3]. K. M. Z. Hasan, S. Datta, M. Z. Hasan, and N. Zahan, ‘‘Automated prediction of heart disease patients using sparse discriminant analysis,’’ in Proc. Int. Conf. Electr., Comput. Commun. Eng. (ECCE), Feb. 2019, pp. 1–6.
[4]. G. Altan, ‘‘Diagnosis of coronary artery disease using deep belief net- works,’’ Makalenizi Yükleyebilmek için Lütfen İngilizce Dilini Seçiniz!!! EJENS, vol. 2, no. 1, pp. 29–36, 2017.
[5]. R. Alizadehsani, M. Abdar, M. Roshanzamir, A. Khosravi, P. M. Kebria, F. Khozeimeh, S. Nahavandi, N. Sarrafzadegan, and U. R. Acharya, ‘‘Machine learning-based coronary artery disease diagnosis: A comprehensive review,’’ Comput. Biol. Med., vol. 111, Aug. 2019, Art. no. 103346.
[6]. G. Luo, G. Sun, K. Wang, S. Dong, and H. Zhang, ‘‘A novel left ventricular volumes prediction method based on deep learning network in cardiac MRI,’’ in Proc. Comput. Cardiol. Conf. (CinC), Sep. 2016, pp. 89–92.
[7]. A. Caliskan and M. E. Yuksel, ‘‘Classification of coronary artery disease data sets by using a deep neural network,’’ EuroBiotech J., vol. 1, no. 4, pp. 271–277, Oct. 2017.
[8]. N. I. Hasan and A. Bhattacharjee, ‘‘Deep learning approach to cardiovascular disease classification employing modified ECG signal from empirical mode decomposition,’’ Biomed. Signal Process. Control, vol. 52, pp. 128–140, Jul. 2019.
[9]. J. Kwon, K. Kim, K. Jeon, and J. Park, ‘‘Deep learning for predicting in-hospital mortality among heart disease patients based on echocardiography,’’ Echocardiography, vol. 36, no. 2, pp. 213–218, Feb. 2019.
[10]. K. H. Miao and J. H., ‘‘Coronary heart disease diagnosis using deep neural networks,’’ Int. J. Adv. Comput. Sci. Appl., vol. 9, no. 10, pp. 1–8, 2018.
[11]. G.-P. Diller, A. Kempny, S. V. Babu-Narayan, M. Henrichs, M. Brida, A. Uebing, A. E. Lammers, H. Baumgartner, W. Li, S. J. Wort, K. Dimopoulos, and M. A. Gatzoulis, ‘‘Machine learning algorithms estimating prognosis and guiding therapy in adult congenital heart disease: Data from a single tertiary center including 10 019 patients,’’ Eur. Heart J., vol. 40, no. 13, pp. 1069–1077, Apr. 2019.
[12]. A. Junejo, Y. Shen, A. A. Laghari, X. Zhang, and H. Luo, ‘‘Molecular diagnostic and using deep learning techniques for predict functional recovery of patients treated of cardiovascular disease,’’ IEEE Access, vol. 7, pp. 120315–120325, 2019.
[13]. P. Lu, S. Guo, H. Zhang, Q. Li, Y. Wang, Y. Wang, and L. Qi, ‘‘Research on improved depth belief network-based prediction of cardiovascular dis-eases,’’ J. Healthcare Eng., vol. 2018, pp. 1–9, May 2018.
[14]. A. Phalke and S. Sonder, ‘‘Deep learning-based heart disease prediction,’’ Asian J. Converg. Technol. (AJCT), vol. 5, no. 1, pp. 1–4, Apr. 2019.
[15]. R. Jin, ‘‘Predict the risk of cardiovascular diseases in the future using deep learning,’’ Ph.D. dissertation, Dept. Elect. Comput. Eng., Univ. Texas San Antonio, San Antonio, TX, USA, 2018
[16]. S. G. van Velzen, M. Zreik, N. Lessmann, M. A. Viergever, P. A. de Jong, H. M. Verkooijen, and I. Išgum, ‘‘Direct prediction of cardiovascular mortality from low-dose chest CT using deep learning,’’ Proc. SPIE, vol. 10949, Mar. 2019, Art. no. 109490X.
[17]. B. Jin, C. Che, Z. Liu, S. Zhang, X. Yin, and X. Wei, ‘‘Predicting the risk of heart failure with EHR sequential data modeling,’’ IEEE Access, vol. 6, pp. 9256–9261, 2018.
[18]. S. Habib, M. B. Moin, and S. Aziz, ‘‘Heart failure risk prediction and medicine recommendation system using exploratory analysis and big data analytics,’’ Ph.D. dissertation, Dept. Comput. Sci. Eng., BRAC Univ., Dhaka, Bangladesh, 2018.
[19]. N. Meng, P. Zhang, J. Li, J. He, and J. Zhu, ‘‘Prediction of coronary heart disease using routine blood tests,’’ 2018, arXiv:1809.09553. [Online]. Available: http://arxiv.org/abs/1809.09553
[20]. C. Krittanawong, K. W. Johnson, R. S. Rosenson, Z. Wang, M. Aydar, U. Baber, J. K. Min, W. H. W. Tang, J. L. Halperin, and S. M. Narayan, ‘‘Deep learning for cardiovascular medicine: A practical primer,’’ Eur. Heart J., vol. 40, no. 25, pp. 2058–2073, Jul. 2019.
[21]. S. M. Awan, M. U. Riaz, and A. G. Khan, ‘‘Prediction of heart disease using artificial neural networks,’’ VFAST Trans. Softw. Eng., vol. 13, no. 3, pp. 102–112, 2018.
[22]. Deep Learning in Science a Survey of Opportunities and Trends. Accessed: Aug. 2019. [Online]. Available: https://towardsdatascience. com/deep-learning-in-science-fd614bb3f3ce
[23]. N.-S. Tomov and S. Tomov, ‘‘On deep neural networks for detecting heart disease,’’ 2018, arXiv:1808.07168. [Online]. Available: http://arxiv. org/abs/1808.07168
[24]. Baccouche, A., Garcia-Zapirain, B., Castillo Olea, C., & Elmaghraby, A. (2020). Ensemble deep learning models for heart disease classification: A case study from Mexico. Information, 11(4), 207.
[25]. S. Tuli, N. Basumatary, S. S. Gill, M. Kahani, R. C. Arya, G. S. Wander, and R. Buyya, ‘‘HealthFog: An ensemble deep learning-based smart healthcare system for automatic diagnosis of heart diseases in integrated IoT and fog computing environments,’’ Future Gener. Comput. Syst., vol. 104, pp. 187–200, Mar. 2020.
[26]. E. Choi, A. Schuetz, W. F. Stewart, and J. Sun, ‘‘Using recurrent neural network models for early detection of heart failure onset,’’ J. Amer. Med. Information. Assoc., vol. 24, no. 2, pp. 361–370, Mar. 2017.
[27]. R. Das, I. Turkoglu, and A. Sengur, ‘‘Effective diagnosis of heart disease through neural networks ensembles,’’ Expert Syst. Appl., vol. 36, no. 4, pp. 7675–7680, May 2009.
[28]. Access on 10-19-2021, available online at: https://archive.ics.uci.edu/ml/datasets/heart+disease.
[29]. T. Helmy and Z. Rasheed, “Multi-category bioinformatics dataset classification using extreme learning machine,” in Proceedings of the IEEE Congress on Evolutionary Computation (CEC '09), pp. 3234–3240, Trondheim, Norway, May 2009.
[30]. S.-J. Wang, A. Mathew, Y. Chen, L.-F. Xi, L. Ma, and J. Lee, “Empirical analysis of support vector machine ensemble classifiers,” Expert Systems with Applications, vol. 36, no. 3, pp. 6466–6476, 2009.
[31]. S. Özşen and S. Güneş, “Effect of feature-type in selecting distance measure for an artificial immune system as a pattern recognizer,” Digital Signal Processing, vol. 18, no. 4, pp. 635–645, 2008.
[32]. H. Kahramanli and N. Allahverdi, “Design of a hybrid system for the diabetes and heart diseases,” Expert Systems with Applications, vol. 35, no. 1-2, pp. 82–89, 2008.
[33]. G. Yan, G. Ma, J. Lv, and B. Song, “Combining independent component analysis with support vector machines,” in Proceedings of the in 1st International Symposium on Systems and Control in Aerospace and Astronautics (ISSCAA '06), pp. 493–496, Harbin, China, January 2006.
[34]. S. Şahan, K. Polat, H. Kodaz, and S. Günes, “The medical applications of attribute weighted artificial immune system (AWAIS): diagnosis of heart and diabetes diseases,” Artificial Immune Systems, vol. 3627, pp. 456–468, 2005.
[35]. W. Duch, R. Adamczak, and K. Grabczewski, “A new methodology of extraction, optimization and application of crisp and fuzzy logical rules,” IEEE Transactions on Neural Networks, vol. 12, no. 2, pp. 277–306, 2001.
[36]. Abdar, M., Książek, W., Acharya, U. R., Tan, R. S., Makarenkov, V., & Pławiak, P. (2019). A new machine learning technique for an accurate diagnosis of coronary artery disease. Computer methods and programs in biomedicine, 179, 104992.
[37]. Firdaus, F. F., Nugroho, H. A., & Soesanti, I. (2021, April). Deep Neural Network with Hyperparameter Tuning for Detection of Heart Disease. In 2021 IEEE Asia Pacific Conference on Wireless and Mobile (APWiMob) (pp. 59-65). IEEE.
[38]. Qrenawi, M.I.; Al Sarraj, W.: Identification of cardiovascular diseases risk factors among diabetes patients using ontological data mining techniques. In: 2018 International Conference on Promising Electronic Technologies (ICPET), pp. 129–134 (2018).
[39]. Awan, S.M.; Riaz, M.U.; Khan, A.G.: Prediction of heart disease using artificial neural network. VFAST Trans. Softw. Eng. 13, 102–112, 2018.
[40]. More, K.; Raihan, M.; More, A.; Padule, S.; Mondal, S.: A12176 Smart phone based “heart attack” risk prediction; innovation of clinical and social approachforpreventivecardiachealth.J. Hypertens. 36, e321, 2018




















