Bioaccumulation Efficacy of Heavy Metals In Body Organs of Rainbow (Oncorhynchus Mykiss) And Brown (Salmo Trutta Fairo) Troutsof Gilgit-Baltistan
Shaukat Ali1*, Javid Hussain2, Salar Ali3, Sarwat Aslam1, Nilma Khan1, Saif Ud Din1
1Department of Environmental Sciences, Karakoram International University Gilgit-Baltistan, Pakistan
2Department of Environmental Science, Baluchistan University of Information Technology, Engineering and Management Sciences, Quetta, Pakistan
3Department of Environmental Science, University of Baltistan, Gilgit-Baltistan, Pakistan
Citation | Ali .S, Hussain.J, Ali.S, Aslam.S, Khan.N, Din.S, “Bioaccumulation Efficacy of Heavy Metals In Body Organs of Rainbow (Oncorhynchus Mykiss) And Brown (Salmo Trutta Fairo) Trouts of Gilgit-Baltistan” International Journal of Innovations in Science and Technology Vol 3 Issue 3 PP 126-141, 2021.
Received | Sep 14, 2021; Revised | Sep 26, 2021 Accepted | Sep 27, 2021; Published | Sep 30, 2021
________________________________________________________________________
Heavy metals are chemical elements that are poisonous and toxic comprising of both necessary and unnecessary trace metals. All aquatic organisms require very low amount of these metals yet in case where these values exceed to certain range, threshold harmful effects are levied upon the ecosystem. The aim of this study was to estimate the bioaccumulation of heavy and trace metals (Cr, Mn, Ni, Fe, Pb, Cu, Cd, Zn) in fish using Atomic Absorption Spectrophotometer (AAS). Samples of fish were procured from Ghizer and Astore districts of Gilgit-Baltistan region of Pakistan. The concentration of Cr, Pb, Cu and Cd were almost same and depicted low tendency of bioaccumulation as per WHO guidelines. The fish from Ghizer was having high concentration of Zn and Fe in intestine. While the concentration of Fe in muscles and intestine from the Astore species was slightly high. The highest concentration of Ni (10.09 ppm) was found in liver tissues of rainbow trout, while the lowest concentration (6.74 ppm) was in the fins of fish from Astore. In case of Cr, the highest concentration (3.8 ppm) was found in liver from both sampling sites, but the lowest concentration (0.24 ppm) was in the muscles of Ghizer Rainbow trout. The highest concentration of cu (6.09 ppm) was in the muscles of fish from Astore, but the lowest concentration (2.32 ppm) was found in many organs of fish from both study sites. Although the concentration of Zn, Mn and Fe were within the limits, however, the highest concentration of Pb (0.79 ppm) was in the muscles and the highest concentration of Cd (0.38 ppm) was in the skin of Ghizer rainbow trout. Concentration of Lead exceeded the limits of FAO/WHO in every organ of fish in both study areas, while all the other metals were in the maximum limits.
Keywords: Bioaccumulation, Brown trout, Fresh water, Heavy metals, Ghizer Rainbow trout
INTRODUCTION
Heavy metals are chemical elements that are poisonous and toxic comprising of both necessary and unnecessary trace metals. All aquatic organisms require very low amount of these metals yet in case where these values exceed to certain range, threshold harmful effects are levied upon the ecosystem [1]. The presence of heavy metals in the tissue of fishes indicate that the aquatic environment is polluted which has been considered a major threat to the aquatic organisms [2].In the last few decades, the rapid development of industry and agriculture has resulted increased heavy metal pollution which is significant environmental hazard for invertebrates, fishes and humans [3].
Fishes are the loftiest consumers in the aquatic food web and their body collects large quantity of heavy metals [2]. Heavy metals being highly persistent and potentially toxic for living organisms, have a particular significance in eco-toxicology [1]. Heavy metals are the well-known pollutants of aquatic environment, and these are accumulated in different tissues of aquatic organisms. Fishes are the most vulnerable aquatic animals of environmental pollution. Therefore, it is necessary to prevent water bodies from accumulating water pollutants. Fishes act as bio-indicators of heavy metal pollution in aquatic environment [4] that are found at high trophic level and are essential source of food [5]. Moreover, fishes are the most important source of protein as well as other essential nutrients for balanced nutrition.
Gilgit Baltistan has enormous freshwater resources. Its rivers, streams and freshwater lakes have a wide range of native and exotic fish species. Twenty different species of freshwater fish are found in the cold waters of GB including 17 native, three exotic and four are endemic species in GB region [6].The exotic species include Rainbow trout. In this region, freshwater resources are the main sources to accomplish major necessities of biodiversity which include glaciers, springs, rivers, ponds and lakes [7]. In GB, fish species are not in large number owing to high water turbidity, speed, low water temperature and low benthic productivity [8].
Major threats for fish in this region are human activities including logging, pesticide use, road construction and diversion of water to irrigation channels [9, 10].The status of heavy metals and contamination in aquatic ecosystems is currently a significant source of concern, because they become poisonous if they exceed permissible limits. Metals enter the aquatic system primarily by air deposition, natural weathering, and anthropogenic activities such as industrial effluents, domestic sewage, and mining waste disposal [11, 12].
Deposition of industrial wastes in rivers, lakes and ultimately into ocean is a significant cause of pollution in aquatic ecosystem. Heavy metal accumulation by aquatic biota can damage tissues which can have adverse consequences in both aquatic organisms as well as in humans [13]. Exposure of aquatic life to the significant amount of heavy metals may result in reduced growth and development, reduction of survival chances of fishes and can disturb the level of oxygen in aquatic system.
Consumption of heavy metals by humans through fishes and other sources may have severe health issues. These metals prove toxic and damage human organs. Effects of toxic heavy metals include nervous disorders, birth defects, gastrointestinal infections, kidney infections, skin lesion, immune system dysfunction and cancer. The physical symptoms of heavy metal assimilation include nausea, abdominal pain, diarrhea, vomiting, chills, weakness and shortness of breath [12, 13].
Furthermore, histopathological changes in various fish tissues are used as indicators of the effects of anthropogenic contaminants and as a reflection of the ecosystem’s health [14]. As a result, there is a growing interest in using fish as biological indicators to assess the integrity and quality of the aquatic environment [15].
The aim of this study is to estimate the concentration of different heavy metals accumulated in different organs of fishes studied in this research. The estimated concentrations are compared with standard values of heavy metals given by WHO and FAO at which aquatic organisms can survive without any health hazard.
Material and Methods
Study Site.
This study was conducted in two districts of Gilgit-Baltistan namely Ghizer and Astore during October 2018 where bio-accumulation of heavy metals in the tissues of rainbow trout was determined. Concentration of heavy metals in Ghizer’s rainbow trout was compared to Astore.
District Ghizer is located in the Hindukash region of Pakistan in the North part of Gilgit-Baltistan, between latitude 36o N to 37oN and longitude 73oE to 74oE having total area of 12042 km2 with the estimated elevation above sea level is 3661 meters. The region is divided into four tehsils i.e. Gupis, Iskoman, punial and Yasin [16]. Iskoman valley is the beautiful and scenic valley of this district which is located at the distance of 140 km from Gilgit capital city and 65 km from Gahkuch. The valley is gifted with natural beauty in the form of alpine meadows, massive glaciers and perennial streams with high mountain valley surrounded by snow caped peaks at the altitude from 7000 to 12000 ft in the Hindukush and Karakorum Ranges while the highest peak near Ishkoman is Shiniki peak that can be visible from Ishkoman Valley.
District Astore is one of the famous places in Gilgit-Baltistan region, which is located in the North of Pakistan. It is located at a distance of 60 km in the Southeast of Gilgit and about 484 km far from Islamabad the capital city of Pakistan. Total area of this district is about 7221 km2 and the total population of this area is 100400.This district is divided into two tehsils i.e. Astore and Shounter. The people of the area depend upon agriculture and livestock farming for their livelihood while they also earn money from seasonal activities. The climatic conditions of the area vary from season to season i.e.in summer season the temperature is not very hot and the overall area has moderate climate, whereas the winters are very cold with heavy snow fall in the region.
Figure1. Map of study area
Sampling of Fish
The fish samples were taken from different fish farms located in the districts. Twenty samples were taken from the districts. Fish were captured from the fresh water of study sites using a hand net with the help of local fisherman. The height and location of study sites were recorded with the help of digital GPS. For further analysis, samples were brought to the research laboratory, Department of Environmental Sciences, Karakoram International University, Gilgit
Laboratory Treatment and Analysis of Fish Samples
Digestion.
Before the analysis of samples, body weight and length of each fish sample were measured with the help of weighing balance. The samples were dissected, and different organs of fish (muscles, intestine, fins, gills, liver, and skin) were separated in the labeled plastic crucibles. Samples were washed thoroughly with distilled water. All the samples were air dried for 24 hours at 10oC temperature in the oven then ground with the help of grinder machine. The fish samples were digested by the method used Podolski [17].This involves digestion of 10g part of ground samples with 10mL HNO3 and 2mL HClO4 and was heated for one hour on a hot plate until all material was dissolved. After complete digestion, the residue was dissolved and diluted with 0.2%V/V HNO3 to 100mL with distilled water. Digested samples were filtered using whatsman filter paper (size). Digestate was stored in a pre-cleaned polyethylene vials until further analysis.
Preparation of stock solution
Standards of Zn, Cu, Fe, Mn, Pb, Ni, Cd and Cr were obtained from the Department of Agriculture & Food Technology laboratory and concentration was made as1000 ppm. Before analysis of fish samples for heavy metals, 100 ppm stock solution was prepared from1000 ppm solution.
Heavy metals in all the digested samples were analyzed at the Hi-tech Laboratory of the Department of Agriculture & Food Technology, Karakoram International University, Gilgit, using Atomic Absorption Spectrophotometer (Varian AA55). Prior to analysis, the instrument was calibrated using standard solutions already prepared in the Laboratory of the Department of Chemistry. The absorption wavelength of the selected heavy metals Zn, Cd, Mn, Pb, Cr, Fe & Ni were noted and results were presented as mean values. The information assimilated for statistical analysis of data was carried out using MS excel in tabulated form afterward transferred to MS word used to evaluate the concentration of heavy metals in the various body organs of fish.
Result and discussion.
Concentration of Heavy Metals in Body Organs of Rainbow Trout Fish. This study showed significant variation of concentration of heavy metals (Cu, Fe,Cr,Cd,Mn,Zn,Ni, Pb) in liver, gills, muscles, intestine, fins and skin of trout fish collected from the areas of Ghizer and Astore. Mean concentration of Zn, Fe, Cr, Cu, Cd, Ni, Pb and Mn in Astore’s fish tissues were in the following range of 7.45-10.09 ppm, 0.26-0.38 ppm, 2.32-6.09 ppm, 2-5 ppm, 0-1ppm,1-6 ppm,0.42-0.62 ppm and 0.25-0.30 ppm, respectively. Figure 2 illustrates the mean concentration of several metals in different organs of fishes studied in two different areas i.e, Astore and Ghizer. The order of heavy metal concentration in the Astore’s fish samples decreased in the sequence for gills as Mn > Cd > Cr > Pb > Fe > Zn > Cu >Ni,for the Muscles as Mn > Cr > Cd > Pb > Zn > Fe > Cu >Ni, for the liver asMn > Cd > Cr > Pb >Zn > Fe > Cu > Ni, for the intestines as Cd > Cr > Pb > Mn >Zn > Cu > Fe> Ni, for the skin as Mn >Cd > Cr >Pb >Fe > Cu > Zn > Ni, for the Fins as Cd > Cr > Pb > Mn > Zn > Cu > Fe >Ni. While the average concentration of heavy metals in the samples, caught from Ghizer were in the range of 6.74-10 ppm, 0.24-0.65 ppm, 2.32-5.25 ppm, 1-12 ppm, 0-1ppm, 1-14ppm, 0.45-0.79 ppm and 0.29-0.38 ppm. Furthermore, the trend of distribution of heavy metals in the Ghizer, fish samples reduced in the sequence for the gills as Mn > Cd > Cr > Pb >Fe > Cu > Zn >Ni, for the muscles as Cr > Cd > Pb > Mn > Fe > Zn > Cu > Ni, for the liver as Mn > Cd > Cr > Pb > Zn > Cu > Fe > Ni, for the intestines as Cr > Cd > Pb > Mn > Cu Ni > Zn > Fe, for the skin as Mn > Cr > Cd > Pb > Fe > Cu > Zn > Ni, for the fins as Cd > Pb > Cr > Mn > Cu > Fe > Zn > Ni.
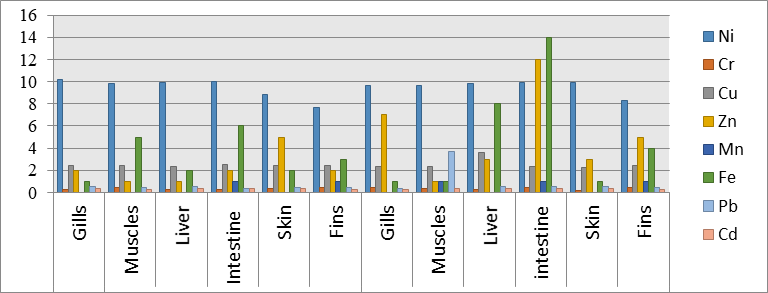
Figure 2. Concentration of heavy and trace metals from Astore and Ghizer regions
The current study revealed the concentration of heavy metals in the different organs of rainbow trout from Astore and Ghizer. The determination of heavy metal concentration in the tissues of fish is a good indicator of pollution status in aquatic environment [18]. The result of this research indicated that all the heavy metals were found in little amount, except Pb which was detected in higher amount in all the parts and tissues of fish procured from both the sampling sites.
Comparison of Heavy Metal Concentration in Various Organs of Rainbow Trout.
Nickel is also an important element and found in soil, air, water and biosphere. This element is essential for living organism, when it is in small amount, but the high amount of nickel is dangerous for human and can cause chronic lung cancer, respiratory failure, nose cancer, asthma, birth defects, and cause many other diseases [19].The sources of nickel emission include mining effluents, domestic effluents, industrial effluents, leaching of heavy metals from solid waste dump and garbage [20].The results of this study investigated that the mean highest concentration on Nickle was found in the liver and skin from both sampling sites (Figure 2a). The highest concentration in liver was (10.09-9.91 ppm), while in skin the concentration was (10-9.95 ppm) from both sampling sites, While the lowest concentration was in the fins (6.74 ppm) of Ghizer rainbow trout. The sequence of distribution of metals in different organs of rainbow trout from both sampling sites were Liver >Skin > Gills >Intestine > Muscle > Fins, although the concentration of Nickle was within the limit of FAO/WHO [21]. Figure 3 shows the mean concentration of copper in different organs of fishes.
Chromium is highly toxic for fish when it enters the body of fish its body surface is covered with mucus, which harms gill epithelium and sudden death is occurred due to suffocation. The traces of chromium in various organs of Rainbow trout from both sites varied from 0.65 to 0.24 ppm (Figure 2b). It was detected that the amount of chromium in different organs was varied from one organ to another from both the Districts. The highest and lowest concentration of Cr was detected in the fins (0.65 ppm) and muscles (0.24 ppm) of Ghizer rainbow trout fish, however the concentration of Cr in all the organs of fishes from both sites were within the limit of FAO/WHO. Uwem et al [22] conducted a study on fresh water fishes in Nigeria, they investigated that the maximum and minimum concentration of Cr that varied from 0.35 μgg-1 to 0.03 μgg-1 in different fresh water fish species.
Table 1.Comparison of heavy metal concentration in different organs of Brown trout from district Astore and Ghizer
|
Metal |
Astore Brown Trout |
Ghizer Brown Trout |
WHO/ FAO (ppm) |
|||||||||||
|
Gills |
Muscles |
Liver |
Intestine |
Skin |
Fins |
Gills |
Muscle |
Liver |
Intestine |
Skin |
Fins |
|||
|
Ni |
10.2 |
9.8 |
9.9 |
10 |
8.8 |
7.68 |
9.67 |
9.64 |
9.81 |
9.9 |
9.95 |
8.34 |
80 |
|
|
Cr |
0.2 |
0.4 |
0.2 |
0.2 |
0.3 |
0.40 |
0.45 |
0.3 |
0.2 |
0.4 |
0.2 |
0.4 |
1 |
|
|
Cu |
2.4 |
2.4 |
2.3 |
2.5 |
2.4 |
2.4 |
2.3 |
2.3 |
3.6 |
2.3 |
2.2 |
2.42 |
2 |
|
|
Zn |
2 |
1 |
1 |
2 |
5 |
2 |
7 |
1 |
3 |
12 |
3 |
5 |
NA |
|
|
Mn |
0 |
0 |
0 |
1 |
0 |
1 |
0 |
1 |
0 |
1 |
0 |
1 |
5.5 |
|
|
Fe |
1 |
5 |
2 |
6 |
2 |
3 |
1 |
1 |
8 |
14 |
1 |
4 |
43 |
|
|
Pb |
0.49 |
0.47 |
0.49 |
0.39 |
0.48 |
0.48 |
0.39 |
3.72 |
0.49 |
0.54 |
0.52 |
0.47 |
1 |
|
|
Cd |
0.3 |
0.2 |
0.3 |
0.3 |
0.3 |
0.2 |
0.3 |
0.4 |
0.35 |
0.3 |
0.3 |
0.2 |
0.2 |
|
Copper is an indispensable element and play avital role in enzyme activity and other cell components having an important role in the formation of hemoglobin [23]. But the abundance of copper in human body is lethal and cause health hazards to human as well as animals. Excessive intake of Cu will be source of food poisoning, nausea, acute stomach pain diarrhea and fever. The National Council Research Center has computed that up to 1.5-3.0 mg daily intake of copper would be safe and adequate [24]. While the current study investigated that the highest concentration of copper was in muscle tissues (6.09 ppm) in the Astore rainbow trout, while in Ghizer rainbow trout the highest concentration of Cu was in Liver (5.25 ppm) (Figure 2c). Liver is the largest organ for the accumulation of heavy metal whereas all the tissues contain the maximum level of copper from both the study areas. The accumulation of copper among the fish tissues was determined in both sites in the decreasing order of Muscle > Liver > Intestine > Fins > Skin > Gills. The permissible limit for cu set by FAO/WHO is (30 ppm), so the concentration of copper in this study was within the permissible limit.
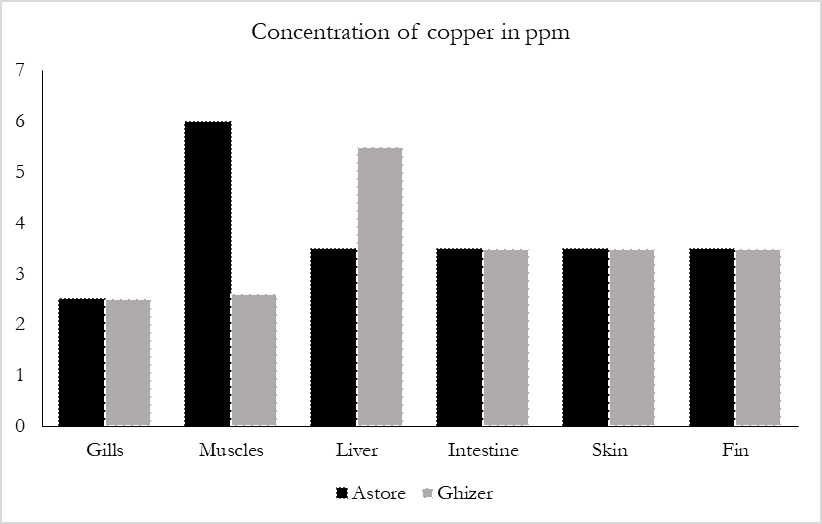
Figure 3: Concentration of copper in different organs of fishes in ppm.
Zinc is also an essential metal and required in a small quantity for a body to perform
a normal metabolic function. When the concentration of Zinc is in excess it is harmful for living organisms as well as fish [25]. However, the data of the current study determined that the maximum concentration was present in intestine (12 ppm) of Ghizer rainbow trout (Figure 2d). While the lowest concentration was in the different organs of fish (1ppm) from both the study areas. The accumulation pattern was in order of Intestine > Gills > Fins > Skin > Liver > Muscles. Concentration of Zinc in the present study was found lower than the permissible limit reported by FAO/WHO.
Manganese is an essential metal and its low level is necessary for human, when it is found in excess amount it can induce oxidative stress and toxic effect in aquatic organism [26]. In this study, the concentration of Mn was not detected in many organs from both districts, while some organs contain a little amount of Mn in intestines, fins and muscles from both areas and the concentration were within the limit of FAO/WHO (Figure 2e). According to [22], concentration of Mn in freshwater fish was below the standard limit of 0.7μgg-1 reported by Charbonneau [27].
Iron is the fourth plentiful component in the earth’s atmosphere. In water, it is present in the form of ferrous or ferric state [28]. It is assumed as one of the essential metals due to its biochemical and physiological role in hemoglobin synthesis and as cofactor of many enzymes [29]. However, high amount of Fe in living organism can increase the level of Fe [30]. The maximum concentration of iron in the current study were (14 ppm) in the intestine of Ghizer rainbow trout, while the lowest concentration was in various organs of fish from both study sites of current study (Figure 2f). The standards set by FAO/WHO are 43 ppm and the results of the current study fall within this limit. So, the current study revealed that lead (Pb) is the only element that exceeds the standards set by FAO/WHO.
Lead is one of the non-essential elements for living organism and can cause neurotoxicity in human being. The result of the current study revealed that the highest mean concentration of Pb was 0.79 ppm, which was found in the muscles of Ghizer rainbow trout fish, while the lowest concentration was in the gill (0.42 ppm) of Astore Rainbow trout (Figure 2g). From both sampling sites the order of Pb accumulation was, muscle > liver >intestine > Skin >fins>gills. WHO suggested that the dietary Pb should not exceed 0.3 μg/g and a recommended limit of 450 μg of Pb per day for adults [31].The concentration of Pb were high in all the organs according to permissible level of FAO/WHO limit (0.3 ppm). Figure 4 shows the concentration of Lead in different organs of fishes in ppm.
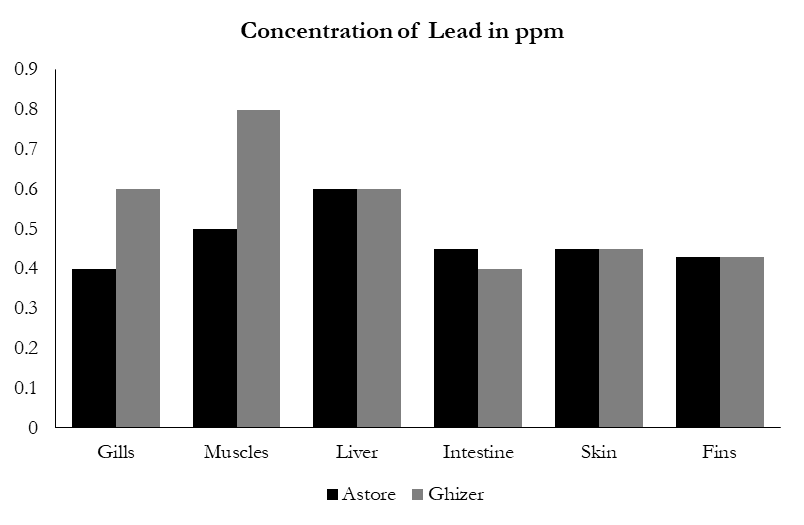
Figure 4: Concentration of lead in different organs of fish in ppm.
Cadmium is the poisonous and non-essential element in food and natural water and mostly this metal is accumulated in the liver and kidney of fishes [32] and is considered lethal and cause chronic lung diseases, kidney damages, high blood pressure and destruction in the formation of red blood cells etc. This study investigated the highest concentration of Cd (0.38 ppm) in the skin of Ghizer Rainbow trout, while the minimum concentration was in the intestine (0.25 ppm) of Astore trout (Figure 2h).However, all the organs of both the sampling sites have maximum level of cadmium which was within the permissible limit of WHO/FAO (0.5ppm).
Concentration of Heavy Metals in Body Organs of Brown Trout Fish.
Heavy metals in fresh water that is used by brown trout (Salmo trutta fairo) in District Ghizer and Astore were assessed. Amount of heavy metals in different parts of Astore trout are summarized in Table 1.Heavy Metal Concentration Comparison in Various Organs of Brown Trout. Heavy metals are formed from both natural and manmade activities [33].In aquatic environment, contamination of heavy metals come out through agricultural discharges, weathering of rocks, municipal discharge, industrial waste etc. [34]. Amount of heavy metals in various organs of Brown Trout is as under:
Nickel is a crucial metal that is present in air, soil and water, also occur in biosphere. Nickel is released into atmosphere through natural cycles and anthropogenic practices. Nickle is accessible in natural environment and it is also released by industries. In the current study, the amount of nickel is maximum in the gills (10.22 ppm) followed by intestine (10 ppm), liver (9.9 ppm), muscles (9.8 ppm), skin (8.8 ppm) and fins (7.6 ppm). Pollutants can also be discharged into the environment by coal and oil-fired power plants [35]. Ni forms complexes with ligands in atmosphere turning into a heavy metal. Whereas, in most organisms nickel is an important component if it is in small quantity. While when its quantity is increased, it becomes poisonous [36]. Nickel can also cause respiratory issues declared by medicinal institution [37].The concentration of Ni falls below the permissible limits of WHO/FAO (80ppm) in all organs of Brown Trout.
Chromium is also a necessary nutrient metal, which is essential for carbohydrates metabolism [38]. Cr enters aquatic environment through different methods like (mining, textiles, metal finishing leather tanneries pharmaceutical industries etc. WHO [39] Reported Lead in close water reservoirs has been as a result of poor treatment of industrial and other effluents, where it is generally present at harmful levels for fish. [40]. Hexavalent chromium and Oxidation state trivalent chromium are stable forms of Cr. Hexavalent chromium has an ability to cross cell membrane and considered to be more poisonous [7].The chromium in all the organs of Brown Trout is found below the set standards of WHO/FAO (1 ppm).
Copper being key constitution of metabolic enzymes, is a vital trace metal for cellular metabolism in organisms [41]. On the other hand, it can be very harmful when it is in high amount and go above the maximum level [42]. Copper Sulphate is used in recreational and commercial ponds to control growth of phytoplankton and filamentous algae and diseases of fish [43]. In the current research, gill and some other tissues of fish copper was detected. Copper is also found below the WHO/FAO (3ppm) guidelines in all organs of Trout Fish.
Zinc is a rich trace metal and is essential heavy metal even if it is present in very low amount in living organism. It is involved in nucleic acid synthesis and present in each cell of the body. It is also involved in many other functions like neurotransmission, immune system and in medicines the Zn its compounds are widely used [44]. Figure 5 illustrates the concentration of zinc in different organs of fishes in ppm. The concentration of Zinc was lower than the permissible limit set by WHO/FAO. Common sources of Zn include galvanized ironwork, zinc chloride used in plumbing and use of Zn containing paints [45].
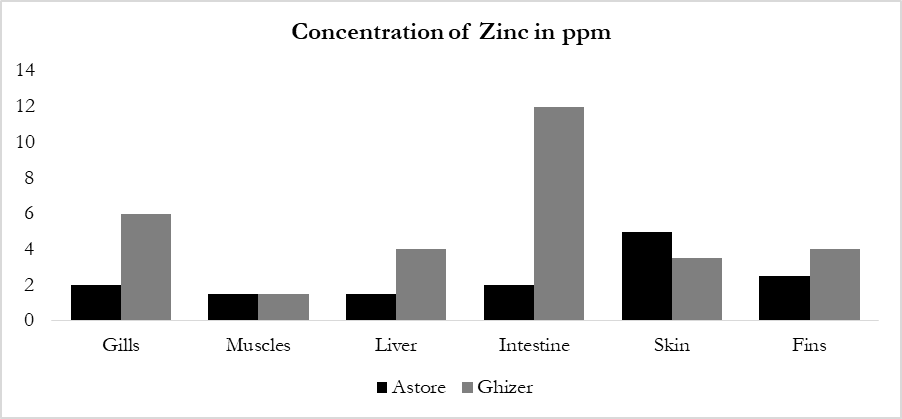
Figure 5: Concentration of Zinc in different organs of fishes in ppm.
Mn can be consumed through water and food resources. Mn does not enter into the skin, and it does not get mixed into the air. As a result, swimming and showering in Mn-containing water does not contribute to increase the exposure. High contact to Mn has been linked with toxicity to the nervous system and syndrome producing. Mn can also be the cause of other diseases like cancer, and it also damage the reproductive system. As compared to the elder, younger children absorb more Mn. So, it is important for children and pregnant women to drink clean water. One of the big problems of water resources is water pollution. Infected rivers have broad impacts; a little manure/chemical fall can have an uneven effect on animals as well as people through harvesting and storage of rainwater in water tanks, runoff may be reduced. The concentration of Mn also falls below the standard set by WHO (5.5 ppm).
Iron is common part of mining and industrial effluents that is released into fresh water. Liver and gonads show the highest concentration of iron while the heart, muscle and liver show the low concentration [46]. In recent times [47], it is found that the mark organ for iron is the fish liver. Feasible method for iron poisoning is physical blockage of gill through accumulation of metals. For the reason that the surface of fish gill tends to be alkaline, respiration is slow down when gills are cover by mucus [48]. Figure 6 illustrates the concentration of iron in different organs of fish in ppm. The high concentration of Fe can cause serious effect on fish by blocking and reducing its gills which spoil the respiratory system consequently it causes sudden death.
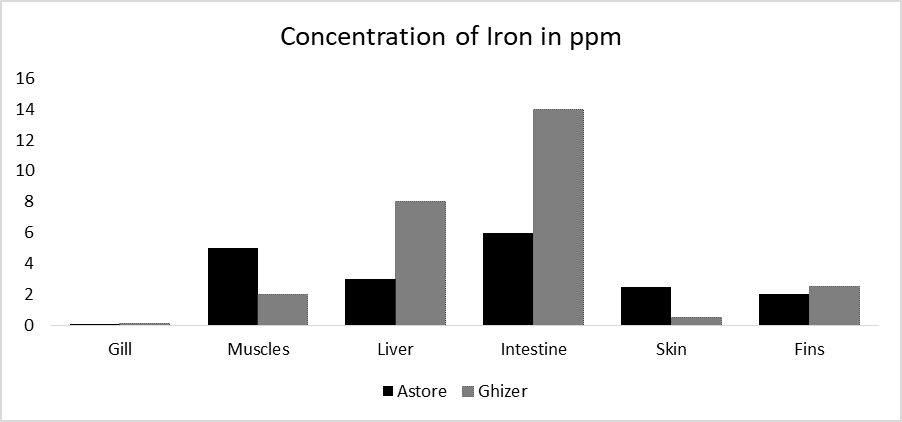
Figure 6: Concentration of iron in different organs of fishes in ppm.
The increased content of iron compound has a significant impact on fish. Fe was found below the permissible limits of WHO/FAO (43ppm) in the body parts of Trout Fish. Concentration of iron in organs of fish is below the WHO permissible limit (4ppm).Lead is a constant and naturally occurring heavy metal. The concentration of lead in environment is increased by anthropogenic activities like (Pb-based paint, manufacturing of mining etc) [49]. Pb may emitted in water through industrial effluents and discharges, when lead dust falls out, through precipitation, through pesticides which contain lead, runoff or through waste water of municipal [50].
The amount of lead is mostly dependent on its absorption into the natural organic matters, into the sediments, on the hardness, alkalinity and pH of water. Aquatic organisms bio-accumulate Pb from water and diet, even though there is proof that Pb deposition is originated from polluted water as compared to food. In gills, kidneys, liver and digestive tract lead is accumulated [51] while the concentration of lead in fish organs is below the WHO permissible limit. While the muscles exceed WHO limit.
Cadmium is nonessential trace metal which occurs naturally. Environmental distress is increased when Cd affinity to bio-accumulate in living organisms up to a dangerous level [52]. During 20 century Cd manufacture, Cd use and emission have increased to the environment considerably, due to industries for example (uses of plastic stabilizers and batteries etc.) and pollution of aquatic life. The contamination of water is also occurred due to the use of fertilizers, pesticides, sewage mud in land, agricultural chemicals which contain cadmium [53, 54]. As a non-degradable increasing impurity, Cadmium is considered capable of changing water trophic levels for centuries. In freshwater fishes, about 75% heavy metals are accumulated in gills, kidney and liver, and also accumulate in hearts and other organs then cause changes in liver, gills and kidney. Figure 7 illustrates concentration of cadmium in different organs of fish in ppm. The cadmium in the body organs of Trout Fish exceeded the standards set by WHO/FAO (0.2 ppm).
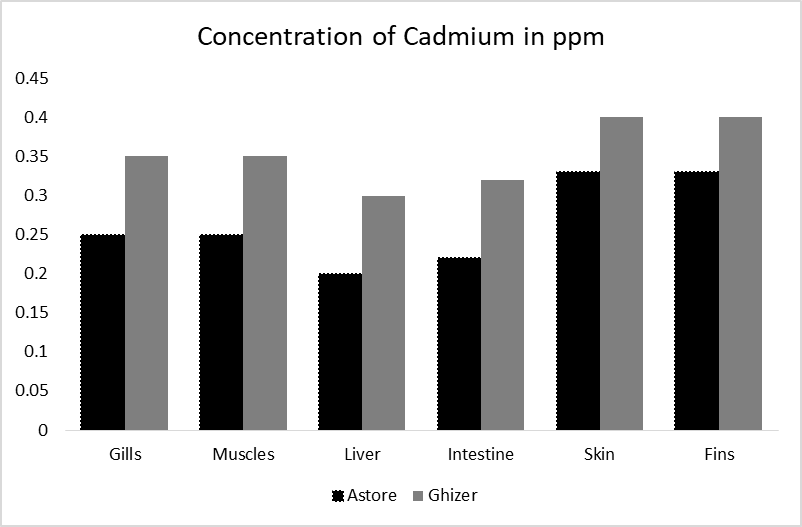
Figure 7: Concentration of cadmium in different organs of fish in ppm.
Conclusion
This study concludes that some heavy metals were accumulated in a few organs of Brown Trout Fish. Results revealed that fish organs contained the maximum level of heavy metals. Concentration of nickel is high in all the organs of Brown Trout from both the sampling areas. Fe and Zn was found higher in the intestine of fish from Ghizer than those from Astore. The concentration of Pb (3.72ppm), Cd (0.37ppm), Cu (2.35 ppm) were exceeded the standard limit set by WHO/FAO.While the concentration of Cu is exceeded from WHO limit (2ppm) in all organs of Brown trout from both sampling areas. This research clearly showed the significant buildup of metals in organs of fish. Consumption of heavy metal contaminated fish should be monitored and avoided to refrain from adversative health effects. Overall contamination of heavy metals was due to natural process but there were certain anthropogenic causes of contamination such as open use of water resources for washing purpose, domestic use and addition of sewage water directly in water resources without proper treatment etc. The concentration of Lead (Pb) dominated all the studied metals and has exceeded the permissible value of FAO and WHO in all organs of fish from both areas of sampling, while all the metals in the various body organs of Astore and Ghizer fishes were within permissible values prescribed by FAO/WHO.
Author’s Contribution. All the authors contributed equally.
Conflict of interest. We declare no conflict of interest for publishing this manuscript in IJIST.
REFRENCES
References
- Ali, S., Begum, F., Hussain,SA.,Khan, SA., Ali, H., Khan,T., Raza, G.,Ali, K., Karim, R. Biomonitoring of HeavyMetals availability in the Marine Environment of Karachi, Pakistan, UsingOysters(Crassostrea ).International Journal of Biosciences, Vol.4, issue 7,pp:249-257, 2014.
- Nair, M., Jayalakshmy, K. V., Balachandran, K. K., & Joseph, T. Bioaccumulation of toxic metals by fish in a semi-enclosed tropical ecosystem. Environmental Forensics, Vol 7, issue 3, pp: 197-206, 2006.
- Authman, M.M.N., Zaki, M.S. Khallat, E.A., Abbas, H.H. Use of fish as bio indicator of the effect of heavy metals pollution. J Aquac Res Dev., Vol 6141,issue 328, 2015.
- Chezhian, N., Kabilan, T., Kumar, T.S., Senthamilselvan. Impact of chemical Factory Effluent on the structural changes in gills of estuarine fish, Mugil cephalus, World Appli Sci J., Vol 9, pp:922-927, 2010.
- Storelli, M.M., et al. Accumulation of mercury, cadmium, lead and arsenic in swordfish and bluefin tuna from the Mediterranean Sea: A comparative study. Baseline / Mar Pollut Bull., Vol 50, pp:993-1018, 2005.
- Govind, P., Madhuri, S. Heavy metals causing toxicity in animals and fishes, Res. J. Anim Vet Fish Sci., Vol 2, issue 2, pp:17-23, 2014.
- BAT, Heavy metal in black sea published by Turkish marine research foundation (TUDAV). Istanbul, Turkey, pp: 71-107, 2014.
- Rafiq, M. Fish Fauna of Gilgit-Baltistan Areas of Pakistan. Final Report Submitted to Gilgit Baltistan Fisheries Department. Pak Mus Nat Hist., pp: 1-114, 2013.
- Ahmed, M.K., Kundu, G.K., Al-Mamun, M.H., Sarkar, S.K., Akter, M.S. Chromium (VI) induced acute toxicity and genotoxicity in freshwater stingingcatfsh, Ecotoxicol Environ Saf., Vol 92, pp:64-70, 2013.
- Rafique, M. Fish diversity and distribution in Indus River and its drainage system. Pak J Zool., Vol 32, pp:321-332, 2000.
- AKRSP/DFID. Development of a Fisheries Strategy for the AKRSP. Draft Consultancy Report No. 14, AKRSP. and Conservation of the Deosai Plateau, Northern Areas, Pakistan. Bioaccumulation of heavy metals in fish tissues of a freshwater lake and Conservation of the Deosai Plateau, Northern Areas, Pakistan. Pp: 27,33-61, 2000.
- Woods, C.A., Kilpatrick, C.W., Rafiq, M., Shah, M., Khan, W. Biodiversity and Conservation of the Deosai Plateau, Northern Areas, Pakistan. Biodiversity of Pakistan. Pakistan Museum of Natural History, Islamabad, Pakistan, pp: 33-61, 1997
- Miretzky, P., Saralegui, A., & Cirelli, A. F. Aquatic macrophytes potential for the simultaneous removal of heavy metals (Buenos Aires, Argentina). Chemosphere, Vol 57, issue 8, pp: 997-1005, 2004.
- Laxmi Priya, S., Senthilkumar, B., Hariharan, G., Paneer Selvam, A., Purvaja, R., & Ramesh, R.. Bioaccumulation of heavy metals in mullet (Mugil cephalus) and oyster (Crassostrea madrasensis) from Pulicat Lake, south east coast of India. Toxicology and Industrial Health, Vol 27, issue 2, pp: 117-126, 2011.
- Yancheva, V., Velcheva, I., Stoyanova, S., & Georgieva, E. Histological biomarkers in fish as a tool in ecological risk assessment and monitoring programs: a review. Applied ecology and environmental research, Vol 14, issue 1, pp: 47-75, 2016.
- Van der Oost, R., Beyer, J., & Vermeulen, N. P. Fish bioaccumulation and biomarkers in environmental risk assessment: a review. Environmental toxicology and pharmacology, Vol 13, issue 2, pp: 57-149, 2003.
- Rahim, I., Ali, S.M., Aslam, M., GIS Based Landslide Susceptibility Mapping with Application of Analytical Hierarchy Process in District Ghizer, Gilgit Baltistan Pakistan. J Geosci Environ Protect. Vol 6, pp:34-49, 2018.
- Poldoski, J.E. Determination of lead and cadmium in fish and clam tissue by atomic absorption spectrometry with a molybdenum and lanthanum treated pyrolytic graphite atomizer. Anal Chem., Vol 52, issue 7, pp:1147–1151, 1980.
- Abarshi, D.A. Bioaccumulation of heavy metals in some tissues of croaker fish from oil spilled rivers. Asian Pac J Trop Biomed., Vol 7, issue 6, pp:563–568, 2017.
- Arya, S., Singh, J., Sharma,H.B. Bioaccumulation in tissues of fresh water fish Cirrhina mrigala on chronic. Int J Environ Rehabil Conser., pp: 1-8, 2012.
- FAO, Review of Heavy Metals. Report No. 22, on the 9 steering committee meeting, Gaborone, Botswana, pp: 13-16, 1996.
- FAO, Cultured Aquatic Species Information Programme. Oncorhynchus mykiss. Fisheries and Aquaculture Department, Cultured Aquatic Species Information Programme, 2012.
- Uwem, G.U. Bioaccumulation of heavy metal in three fresh water fishes caught from cross. European J Exp Biol., Vol 3, issue 3, pp:576-582, 2013.
- Sivaperumal, P., Sankar, T.V., Nair, P.G.V. Heavy metal concentrations in fish, shellfish and fish products from internal markets of India vis-`a-vis international standards. Food Chem. Vol 102, pp: 612-20, 2007.
- Li, Lan., Zhang, K., Gill, R.A., Islam, F., Farooq, M.A., Wang, J., Zhou, W. Ecotoxicological and Interactive Effects of Copper and Chromium on Physiochemical, Ultrastructural, and Molecular Profiling in Brassica napusBioMed pp: 17, 2018.
- Ardakani, S.S., Jafari, S.M. Assessment of heavy metals (Cu, Pb and Zn) in different tissues of common carp (Cyprinuscarpio) caught from Shirinsu Wetland, Western Iran J Chem Health Risks. Vol 4, issue 2, pp:47-54, 2014.
- Vieira, M.C., Torronteras, R., C´ordoba, F., Canalejo, A. Acute toxicity of manganese in goldfish Carassiusauratus is associated with oxidative stress and organ specific antioxidant responses. Ecotox Environ Safe. Vol 78, pp :212-700, 2011.
- Charbonneau, C.S., Nash, T. Contaminants program, Mingo National Wildlife Refuge (Region 3), contaminants survey results. U.S. Fish and Wildlife Service, 1993.
- Ghulman, B.A., EL-Bisy, M.S., Ali, H. Ground water assessement of makkah al-mokarama. Proceedings of the 12th International Water Technology Conference, Umm Al-Qura University, Makkah, pp: 1515-1527, 2008.
- Gorur, F.K., Keser, R., Akcay, N., Dizman, S. Radioactivity and heavy metal concentrations of some commercial fish species consumed in the Black Sea Region of Turkey. Chemosphere, Vol 87, pp:356-61, 2012.
- Edward, J.B., Idowu, E.O., Oso, J.A., Ibidapo, O.R. Determination of heavy metal concentration in fish samples, sediment and water from Odo-Ayo River in Ado-Ekiti, Ekiti State, Nigeria. Int J Environ Monit Anal., Vol 1, issue 1, pp:27-33, 2013.
- Chi, Q.Q., Zhu, G.W., Alan, L. Bioaccumulation of heavy metals in fishes from Taihu Lake, China. J Environ Sci., Vol 19, pp:1500–1504, 2007.
- Velayatzadeh, M.J. (2013) Determination of heavy metals and trace elements in the muscles. J Anim Plant Sci., Vol 23, issue 3, pp:786-791, 2013.
- Bauvais, C., Zirah, S., Piette, L., Chaspoul, F., Domart-Coulon, I. sponging up metals: Bacteria associated with the marine sponge Spongiaoffcinalis. Mar Environ Res., Vol 104, pp: 20-30, 2015.
- Demirak, A., Yilmaz, F., Levent, Tuna, A., Ozdemir, N. (2006) Heavy metals in water,sediment and tissues of Leuciscus cephlaus from a stream in southwesternTurkey. Chemosphere, Vol 63, pp: 1451-1458, 2006.
- ATSDR (Agency for Toxic Substances and Disease Registry), Agencyfor Toxic Substances and Disease Registry, Division of Toxicology, CliftonRoad, NE, Atlanta, GA, 2004.
- Friedrich, A.R., Filice, F.P. Uptake and accumulation of the nickel ion byMytilus edulis. Bull. Environ. ContamToxicol., Vol 16, pp:750-755, 1976.
- Nielson, F.H. (1977) Nickel toxicity. John Wiley and Sons Inc, New York.
- Pacheco, M., Santos, M.A., Pereira, P., Martínez, J.I., Alonso, P.J., Soares, M.J.,Lopes, J.C. EPR detection of paramagnetic chromium in liver of fish(Anguilla anguilla) treated with dichromate(VI) and associated oxidative stress responses- Contribution to elucidation of toxicity mechanisms. Comp BiochemPhysiol C., Vol 157, pp:132-140, 2013.
- WHO (World Health Organization), Chromium, Nickel and Welding.International Agency for Research on Cancer. IARC Monographs on theEvaluation of Carcinogenic Risks to Humans, France, 1990.
- Eisler, R. 2000. Handbook of chemical risk assessment: Health hazards tohumans, plants, and animals.
- Michael, P., (1986) Ecological methods for field and laboratory investigations.TATA McGraw-Hill Publishing Company Ltd., New Delhi.
- Boyd CE (1990) Water quality in ponds for aquaculture. Alabama AgriculturalExperiment Station, Auburn University, USA.
- Arellano, J.M., Storch, V., Sarasquete, C. Histological changes and copperaccumulation in liver and gills of the Senegales Sole, Solea senegalensis.Ecotoxicol Environ Safe., Vol 44, pp:62-72, 1999.
- Alabaster, J.S., Lloyd, R., Water quality criteria for freshwater fish. Second edition. London, FAO. Pp: 456, 1982.
- Farombi, E.O., Adelowo, O.A., Ajimoko, Y.R., Biomarkers of oxidative stressand heavy metal levels as indicators of environmental pollution in African catfish (Clariasgariepinus) from Nigeria Ogun River. Int J Environ Res PublicHealth., Vol 4, pp:158-165, 2007.
- Abbas, H.H., Zaghloul, K.H., Mousa, M.A.) Effect of some heavy metalpollutants on some biochemical and histopathological changes in Blue tilapia,Oreochromis aureus. Egypt J Agric Res., Vol 80, pp:1395-1411, 2002.
- Omar, W.A., Saleh, Y.S., Marie, M.A.S) Integrating multiple fish biomarkersand risk assessment as indicators of metal pollution along the Red Sea coastof Hodeida, Yemen Republic. Ecotoxicol Environ Saf. Vol 110, pp :221-231, 2014.
- Peuranen, S., Vuorinen, P.J., Vuorinen, M., Hollender, A. The effects of iron,humic acids and low pH on the gills and physiology of Brown Trout (Salmotrutta). Ann Zool Fennici. Vol 31, pp: 389-396, 1994.
- Cretì, P., Trinchella, F., Scudiero, R.) Heavy metal bioaccumulation andmetallothionein content in tissues of the sea bream Sparus auratafrom threedifferent fish farming systems. Environ Monit Assess., Vol 165, pp:321-329, 2010.
- Katti, S.R., Sathyanesan, A.G.) Lead nitrate induced changes in lipid andcholesterol levels in the freshwater fish Clariasbatrachus. Toxicol Lett., Vol 19, pp:93-96, 1983.
- Fernandez, P, J., Roman, A., De las Rivas, J., Bustelo, X.R., Dosil, M.) The 90S preribosome is a multimodular structure that is assembled through a hierarchical mechanism. Mol Cell Biol., Vol 27, pp:5414–5429, 2007.
- Melgar, M.J., Perez, M., Garcia, M.A., Alonso, J., Miguez, B. The toxic andaccumulative effects of short-term exposure to cadmium in rainbow trout (Oncorhynchus mykiss). Vet Hum Toxicol., Vol 39, pp:79-83, 1997.
- Ali S., Rubina, Hussain S. Assessment of Freshwater Springs, Associated Diseases, and Indigenous Perception in District Ghizer, Pakistan. Pak J Med Sci.; vol 34, issue 1, pp:121-124, 2018.




















