Degradation of Bioplastics under the Influence of Several Environmental conditions
Muhammad Rizwan1 Tabasam Jamal2
1 Government College University Lahore
2Department of Geography, Punjab University, Quid e Azam Campus, Lahore.
* Correspondence: rizwanm694@yahoo.com
Citation | Rizwan. M and Jamal.T, “Degradation of Bioplastics under the Influence of Several Environmental conditions”. International Journal of Innovations in Science & Technology, Vol 03 Issue 03: pp 93-101, 2021.
Received |May 30, 2021; Revised | June 22, 2021; Accepted | July 04, 2021; Published | July 08, 2021.
________________________________________________________________________
Abstract.
The increasing threats of plastics to the natural environment encouraged the production of bio-plastics from renewable biomass resources. The premium quality of bio-plastics are mainly produced by treating starch with glycerol. Plastics are basically non-biodegradable synthetic or semi synthetic products. This study aims at analyzing the degradation patterns of bio-plastics. The bio-plastics are ecologically less toxic than the synthetic plastic materials. The bio-plastics can degrade in several environmental conditions including aquatic environment, compost and soil. The bioplastic materials are buried in composite soil or loam sand to analyze degradation activity by taking photographic data and measuring the weight. Effect of weather conditions on the degradation activity was analyzed by recording different weather conditions including temperature, humidity, rainfall sunshine intensity and duration of sunlight. The comparative results portrayed the degradation activity of bio-plastics which was accomplished through hydrophilic enzymes. The initial regenerating material absorbs moisture of soil after saturation and the weight was increased up to 87%. The weight of bio-plastics reduced steadily after the initiation of decomposition. Invasion of soil microorganisms enhance the degradation activity. The environmental features including rainfall, humidity and sunlight intensity also affects the disintegration of bioplastics. The increased intensity of sunshine increased the microbial activity of soil which in turn increased the rate of degradation of bio-plastics.
Keywords: Bioplastic, Degradability and Purified Glycerol.
1. Introduction
Plastics are widely used commercial products made of synthetic and semi synthetic raw materials. Plastics are non-bio-degradable or take centuries to degrade under natural climatic conditions [1]. These are light, versatile, moisture resistance, tough and cost efficient which are commercially used for packaging, textiles and in electronic devices all over the world. Plastics are considered non-biodegradable because it disintegrates in a period beyond human time frame [2]. The petroleum-based plastics have several drastic effects on environment and one of them is destruction of natural beauty of landscape [3]. Persistence of plastics in soil alters the structure of soil and destroys the biota of soil. Littering of plastics in water bodies are a major cause of death of aquatic organisms including zooplanktons, mammals, fish, amphibians and birds depending upon water for fee. Ingestion of any plastic material can cause death of organisms for instance, fish, turtles, whales and birds [4, 5, 6]. The plastics are the most hazardous materials for food chain, and can block drainage channels and sewerage lines if these are not properly disposed off causing breeding of mosquitoes and other vectors in ground which can spread diseases. Thus, this blockage aggravates the chance of floods and cause problems related to waste management [7].
Thus, due to increasing environmental hazards there is a dire need of renewable or degradable packaging materials. One great effort to decrease the life span of petroleum based plastics was to blend these with natural polymers for instance starch. This blending converted the petroleum plastics into bio-plastics. Other polymers including lignin, protein resins and cellulose has also been used to develop bio-plastics [8]. Lipids and animal fats are also used to produce bio-plastics. Particularly the major carbohydrate used for manufacturing bioplastics is obtained from cereals and plant tubers. Starch is abundant and cost efficient naturally but plasticizing increases its mechanical properties including flexibility [9]. Glycerol is used as a plasticizing agent for the breakdown of hydrogen bonds formed between the monomers of starch granules constituting a polymer. Bioplastics including starch blends with biodegradable polyesters like Polylactic acid (PLA), Polycaprolactone & Ecoflex and Thermal plastic starch (TPS) which are high quality bio-plastics manufactured by starch [10]. Glycerol is produced along biodiesel as a byproduct, its direct disposal is hazardous for the natural environment but it has multiple applications in pharmaceutical, polymers, food and cosmetic industry after purification. Using glycerol plasticizer is quite beneficial as it manufactures less pollutant and environment friendly bio-plastics. The bioplastics are introduced as an alternative to non-renewable petroleum based plastics due to their ability to decompose in shorter time as compared to petroleum based plastics. Bioplastics decompose through microbial activity of soil. In soil, microorganisms produce enzymes under optimal conditions to degrade bioplastics [11].
Different factors affecting the decomposition activity of bioplastics including pretreatment, microorganisms and characteristics of polymer. The general characteristics of polymers include molecular weight, mobility, and nature of plasticizers, tactility and crystallinity. The decomposition activity usually takes place in optimal conditions including humid conditions because hydrophilic enzymes usually occur in moist environment. In soil, various organisms including bacteria, fungi or worms secrete amylase enzyme in external environment which breaks down starch [12]. This enzyme degrades the insoluble starch materials to soluble products which are usually absorbed by microbial cells, these products include maltose and glucose. During decomposition, the large polymers are degraded into monomers by breaking hydrogen bonds through enzymes, thus viscosity is reduced and the product is liquefied [13]. Different saccharides are formed in a process known as scarification following the breakage of bonds. The microbial activity of soil is increased in the presence of water, oxygen, temperature and light intensity.
Thus the bioplastics readily degrade in presence of water. The bioplastics are usually coated on paper bags to increase the stability of paper bags and to retain oil and moisture [14, 15, 16].
This study aims at investigation of sequential decomposition/degradation/disintegration of bioplastics at various environmental conditions.
2. Materials and Methods
Study area
This research was done in coastal agricultural region of Karachi during the year 2020. The temperature of this region is high and overall climate is humid. Cassava roots are harvested in the fields to obtain starch [17,18].
Material and method
Starch is used to manufacture bioplastics using glycerol as plasticizing agent and bioplastics are hydrolyzed using acetic acid or hydrochloric acid. Peels of cassava roots are rich in starch. To obtain starch cassava, roots were treated with 0.1 M NaCl to extract the peel using a warring blender. The homogenate was strained through a muslin cloth. The homogenate was dried in oven for 24 hours at 60˚C. Starch was plasticized with glycerol, while glycerol is of two types i.e, Analytical Grade (AG) obtained from local area and purified glycerol obtained from vegetable oil [19].
Reagents
Various reagents were used along glycerol; these reagents include Acetic acid, Nacl, HCl and AG glycerol. For plasticization, 450ml distilled water was added into 35g starch in order to form a mixture to which 0.1M acetic acid (15g) and 10ml of glycerol was added. This mixture was then heated for 45 minutes at 120˚C in a 850ml beaker. This mixture was then poured into a tray after cooling. The mixture was spread on tray with the help of rod. The tray was again heated for 18 hours at 60˚C. The dried mixture was termed as bioplastics which cut into smaller pieces for laboratorial use [20, 21].
Burying of bioplastics
Before burying bioplastics, the initial weights of pieces was measured. The effect of decomposition was measured by taking photographs and measuring weight of buried bioplastics weekly. In laboratory, bioplastics were decomposed with three methods; in first method, bioplastics were submerged in 100ml of distilled water and poured in a 250 ml beaker at room temperature. In second method, the bioplastics were buried in soil nearly 3 inches deep. In this soil almost 6ml of water was added weekly to retain the moisture level [22, 23]. In the third method bioplastics were observed under natural environment. Then these bioplastics were covered with polyester and buried in 30cm deep soil. Effects of changing weather conditions including rainfall, temperature and humidity are also recorded.
Table 1: Reagents used in Bioplastic Samples
|
Samples |
Starch (g) |
Water (ml) |
Acid |
Glycerol |
|
Bioplastic 1 |
35 |
450 |
HCL |
Analytical Grade (AG) |
|
Bioplastic 2 |
35 |
450 |
HCL |
purified glycerol |
|
Bioplastic 3 |
35 |
450 |
Acetic acid |
purified glycerol |
3. Results
The disintegration period of three bioplastics is given in the Table 2. The physical appearance of bioplastics changed when decomposition started. The thickness of bioplastics increased due to absorption of water which darkened the color of bioplastics. Following this, the bioplastics degraded into smaller components until these were completely disintegrated. The disintegration enables the microorganisms to attach to bioplastic components and digest starch. By the fifth week of decomposition, the bioplastics were disintegrated to very small brittle components.
Change in Bioplastic Weight
Weight of buried bioplastics changed by the time. The general trend of decomposition involve increase in weight, further variations in bioplastics due to soil organisms and decrease in weight following the complete disintegration of bioplastics. The initial increase in weight is due to absorption of water in bioplastics by hydrophilic enzyme to start disintegration. As starch is hydrophilic, therefore its weight varies according to the moisture content of soil which in turn depends upon different environmental conditions including rain. After moisture absorption, the microorganism in soil completely degrade the bioplastics. Moisture content plays a significant role in the disintegration of bioplastics as the starch itself and enzyme is hydrophilic as well as microbial activity of soil also require moisture. The weight of bioplastics decrease when microorganisms decompose the bioplastics after three weeks. The first bioplastics were digested by worms; the second one was ingested by termites while the third bioplastics were disintegrated by fungi, all these bioplastics were completely broken down within 10 weeks. There was no significant variation in weight of bioplastics hydrolyzed with local glycerol or acetic acid unlike those who were hydrolyzed with HCL.
Table 2: weekly physical changes in weights of bioplastics under the influence natural environment.
|
Change in weight |
Week 1 |
Week 2 |
Week 3 |
Week 4 |
Week 5 |
|
Bioplastic 1 |
8.03g |
11.57 g |
12.19g |
12.5g |
7.06 |
|
Bioplastic 2 |
8.03g |
18.74g |
19.05g |
17.06g |
16.89g |
|
Bioplastic 3 |
8.03g |
9.09g |
10.94g |
9.14g |
5.48g |
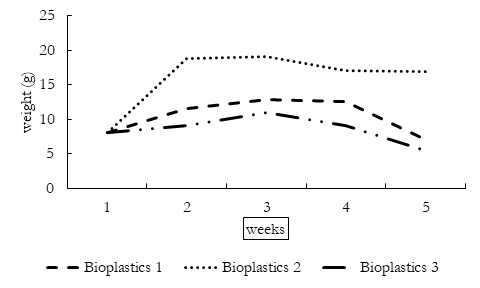
Figure 1. Change in Bioplastic weight over five weeks of degradation period.
The HCL is strong acid which requires massive moisture content to disintegrate into water and free ions which possibly causes the weight of bioplastics to vary greatly.
Effect of Weather on Bioplastic Degradation
During ten weeks of decomposition, the following weather conditions were recorded; average temperature was from 20˚C to 43˚C, sunshine hours ranged from 0.0 - 12 hrs, average rainfall ranged from 0.0 to 43 mm and relative humidity ranged from 61.5% to 84.5%. Rainfall is closely related to the variations in weight of bioplastics as the rainfall increases the content of water in soil and facilitates the disintegration of bioplastics. For the first bioplastics, temperature play significant role in variation of weight while sunshine hours has positive correlation only with the third bioplastics. While relative humidity had an inverse relation with weight of bioplastics.
Rainfall & Sunshine Hours Contribution to Degradation
The decomposition of bioplastics started with low rainfall volumes i.e, 2.3mm. During first two weeks the weight of bioplastics increased with decreasing rainfall volume. After two weeks, the weight of bioplastics drastically increased when the rainfall volume increased up to 0.05mm. After four weeks, the weight started decreasing due to decreasing rainfall and precipitation rate. Rainfall increases the moisture content of soil which facilitates degradation of bioplastics. Thus rainfall provides optimal conditions for the disintegration of bioplastics through hydrophilic enzymes.
The rate of weight loss was reduced during 4th to 7th week when rainfall level decreased significantly. After the decomposition of larger components, the smaller components created high volume to surface ratio for moisture absorption.
The sunshine affects the microbial activity of soil, facilitates the biological activity and breakdown of biological molecules. The weight of bioplastics increases by the reduction of sunshine. Moderate sunshine expedites the breakdown of bioplastics in soil facilitating the hydrophilic enzymes resulting in an increased decomposition of bioplastics.
Laboratory Degradation Trend for Bioplastics in Water
The weight of bioplastics soaked in water increased during first three weeks. The weight of bioplastics declined from 4th to 8th week during which the substrate was completely dissolved and disintegrated.
Table 3: Weekly physical changes in weights of bioplastics when the substrate is completely dissolved.
|
Change in weight |
Week 5 |
Week 6 |
Week 7 |
Week 8 |
Week 9 |
|
Bioplastic 1 |
7.06g |
6.28g |
5.09g |
4.95g |
4.06 |
|
Bioplastic 2 |
16.89g |
12.74g |
11.05g |
10.06g |
8.89g |
|
Bioplastic 3 |
5.48g |
5.09g |
4.94g |
3.95g |
3.18g |
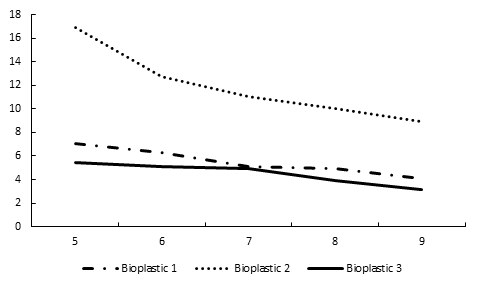
Figure 2: Change in Bioplastic weight from five weeks of degradation period.
The third type of bioplastics were hydrolyzed with acetic acid and decomposed in a shorter time as compared to first two bioplastics. The bioplastics soaked in distilled water had no significant differences.
The laboratory experiments of bioplastics indicated that pure glycerol based bioplastics take less time in decomposition. These experiments suggest that hydrolyzing agents and purity of glycerol affects the rate of decomposition of bioplastics in any medium.
4. Discussion
Plastics made by renewable plant based materials are termed as bioplastics. These plastics can be obtained from natural resources as well as can be synthesized chemically in laboratories. One of the major biological molecules used for the production of bioplastics is starch. Bioplastics have advantage over petroleum based plastics that they can degrade in shorter time as compared to the petroleum based bioplastics. The pattern of degradation is necessary to understand the time and factors required for the decomposition of plastics and to have an insight of materials used to manufacture plastics with desirable rate of decomposition. The process of decomposition initiates with the hydrolysis of bioplastics and followed by the breakdown of polymers into monomers which are absorbed by microorganisms. Microbial activity of soil is carried out with the help of soil organisms including worms, bacteria, worms and fungi. The decomposition process is facilitated by the soil organisms which may or may not be visible to the human eye. The microbial activity starts in the second week following the increase in weight of substrate and then the weight decreases by microbial activity.
Natural process of decomposition is more effective in comparison to the enzymatic decomposition. Different types of soil comprises of different composition and microbes to facilitate decomposition. The rate of decomposition is doubled in moist and porous soil which hosts greater number of microbes. The rate of degradation of first bioplastics is high and its assimilation period is short. The microorganisms also decrease when the substrate becomes dehydrated. Reduction of rainfall decreases the moisture content of soil and rate of decomposition is also reduced.
The mechanical properties of bioplastics also contribute to the rate of decomposition. The degradation rate of bioplastics is lower with high tensile strength. However, the decomposition rate in water was same for bioplastics with varying mechanical strength.
5. Conclusion
Petroleum based plastics are hazardous for natural environment due to their long degradation period. Thus, fast decomposing material such as starch should be used as raw material for manufacturing of bioplastics. Starch being hydrophilic, decomposes faster in aqueous medium as compared to soil. The bioplastics can also decompose within few weeks in well moist healthy soil with multiple microorganisms. Bioplastics manufactured from starch produced from cassava peel plasticized with glycerol can be decomposed completely within ten weeks under microbial activity of soil. Different environmental factors also affect the degradation activity of bioplastics.
Conflicts of Interest
The author/s declare no conflicts of interest regarding the publication of this paper.
References




















