Evaluation of Microbial Contamination via Wastewater Collected from Different Oil Industries and its Treatment Using Various Coagulants
Saman Basharat 1, Sumaira Mazhar 1*, Roheela Yasmeen 1*, Wajeeha Hamid 1
1Department of Biology, Lahore Garrison University, Sector C, DHA Phase VI, Lahore, Pakistan.
*Correspondence: sumairamazhar@lgu.edu.pk, roheelayasmeen@lgu.edu.pk
Citation | Basharat. S, Mazhar. S, Yasmeen. R, Hamid. W. “Evaluation of Microbial Contamination via Wastewater Collected from Different Oil Industries and its Treatment Using Various Coagulants”. International Journal of Innovations in Science and Technology. Vol 4, Issue 2, 2022, pp: 392-403
Received | March 25, 2022; Revised | April 20, 2022; Accepted | April 23, 2022; Published | April 25, 2022.
________________________________________________________________________
Wastewater from industrial discharged into other water bodies that pose serious risks to human health as well as the environment. The oil and ghee industries are also the main contributors to water pollution along with various other industries. The present study aimed to evaluate microbial load in waste water of oil industries in Lahore and its treatment using chemical and natural coagulants. Water samples were collected from three selected oil and ghee industries in Lahore. Physicochemical properties (Chemical oxygen demand (COD), Biological oxygen demand (BOD), and turbidity) and microbial contamination of water samples were analyzed before and after treatment. It was observed that samples treated with natural coagulants such as orange and banana peel, and date seeds showed a mild reduction in physicochemical parameters. Orange and banana peel coagulants caused a 30% reduction, while date seeds coagulants caused a 60% reduction in physicochemical parameters. A significant decrease in microbial load was noticed by using natural coagulants. However, for the chemical coagulants, it was observed that ferric chloride with alum and Ca+2 cation with bleaching powder caused an extreme reduction in physicochemical indicators and microbial load. While no significant decrease was observed in physicochemical indicators and microbial load when waste water samples were treated with Poly Aluminum chloride (PAC) and alum. It was concluded that chemical coagulants have a better ability to treat waste water as compared to natural coagulants.
Keywords: Wastewater treatment, natural coagulants, chemical coagulants, microbial contamination.
INTRODUCTION
Water is essential for human life and the maintenance of the ecosystem. Water scarcity and poor water quality are two main issues raised by improper water use. Water pollution is an alarming condition and may cause many communicable and non-communicable diseases. Industrial waste water is the main cause of water pollution as water is discarded in the environment without being treated [1]. These industries include the oil industry [2], paper industry [3], brewery industry [4], cosmetic industry [5], and paint industry [6].
Water pollution is indicated by physical, chemical, and biological indicators that describe the quality of water. Increased Biological Oxygen Demand (BOD), Chemical Oxygen Demand (COD), turbidity, and microbial load indicate the poor Water quality. Untreated water can contaminate the downstream areas and cause several diseases by direct and indirect means [7].
Many physical, chemical and biological processes are being applied to treat waste water. Chemical treatment is most common in this regard. Among all the chemical treatments, coagulation is mostly used for water treatments in industrial plants as these are simple and cost-effective options [8]. Chemical-based coagulants are being used to treat waste water. Inorganic coagulants like alum, calcium hydroxide, ferric chloride, ferrous sulphate, Poly aluminum chloride (PAC), etc., are commonly used coagulants for water treatment. These coagulants are effective as they reduce the BOD, COD, turbidity, and microbial load in the water. However, the high cost and toxic effects of chemical coagulants pose a risk to human health. A large amount of sludge at the end of the process is another point of concern [9]. Natural coagulants have been considered for water treatment without any harmful effects for ages. Natural coagulants are cost-effective, biodegradable, and non-toxic. Opuntia mucilage, Psyllium husk, Trigonella foenum-graecus, Dolichos lablab, Hibiscus Rosa Sinensis, Banana juice, and Roselle seeds are some well-known natural coagulants for water treatment [10].
Ghee and oil industries are the main cause of waste water pollution in Pakistan. Oil, grease, toxic elements, BOD, COD, Total suspended solids (TSS), and Total dissolved solids (TDS) are common pollutants released from oil and ghee industries. These pollutants are harmful to human health and the environment [5]. A scarce data is reported on oil and ghee industries waste water management. Therefore, the present study aimed to evaluate the physicochemical parameters and microbial contamination in waste water of oil and ghee industries in Lahore. Moreover, it compared the two treatment methods such as natural and chemical coagulants that are used to treat waste water samples purposefully to find the best remedy that can be adopted to reduce BOD, COD, turbidity, and microbial load.
MATERIALS AND METHODS
Sample collection
A total of Three samples (n=3) were collected from the effluents of Khalis Group of Industries (KGI), Hamza Vegetable Oil Refinery, Ghee Mills (HVO), and Momin Cooking Oil and Ghee Mills (MCO) located in Lahore in January 2021. Samples were collected from the discharging processing outlet of the industries in the pre-sterilized polythene bottles. The samples were kept at a temperature of 25ºC to maintain the growth of microbial contamination. Sample codes allocated to each industrial sample were labeled as KGI, HVO, and MCO.
Determination of physicochemical parameters
Physiochemical parameters (odor, temperature, pH, turbidity, COD, and BOD) of collected waste water samples were recorded. The centigrade thermometer noted the temperature of all the collected wastewater samples. A pH meter (HANNA PH210) was used to measure the pH of the samples. The Nephelometric method was used to calculate the turbidity of wastewater samples through a turbidity meter (HANNA HI93703). APHA standard methods were used for the manual calculation of the COD for the present studied samples through the closed reflux method and titrimetric method [11].
Microbial determination
The samples then proceeded for bacterial analysis. All three wastewater samples were tested for Total Plate Count (TPC) by applying the pour plate method on Luria-Bertani (LB) agar [12]. Violet red bile lactose agar was used to detect and enumerate lactose fermenting coliform microorganisms [13]. Eosin methylene blue agar was used for the identification of E. coli [14].
The CFU/mL was calculated using the formula:
CFU/mL= (no. of colonies x dilution factor)/volume of culture plate
Preparation of 2% orange peel and 2% banana peel coagulant
Organic banana and orange peels were air-dried and placed in a drying oven at 90ºC for a day. The dried fruit peels were separately crushed into powder with the help of a pulverizer. Nearly 2.5 grams of orange and banana peels powder were weighted separately and mixed. Sodium hydroxide solution was added to destabilize the colloidal substances. The solution was stirred by a shaker for 5 min at medium speed. The solution was placed in the water bath at 60ºC, and it was allowed to leach for 15 min. Different doses were prepared to use both banana and orange peels powder in combination. 15 mg of orange and banana peels powder was dissolved in 1000 mL water and agitated in a shaker at medium speed. 25mg, 35mg, and 45 mg doses of orange and banana peels powder were prepared.
Preparation of 5% date seeds coagulant
Date seeds were washed and dried at 55°C for 10 h to remove the excess moisture and then were burnt to convert them into activated carbon. The activated carbon seeds were dipped in sulfuric acid overnight and washed thoroughly with distilled water. The seeds were dried for a day at room temperature. The dried seeds were crushed and powdered by using a grinder machine. The 5% stock solution of date seeds powder was prepared. The prepared coagulant was placed in a shaker at medium speed for 5 min.
5% Alum used in combination with 1% PAC
Different doses were prepared to use both alum and PAC in combination. 15 mg of PAC and 5 mg of alum were dissolved in 1000 mL water and agitated in a shaker at medium speed. After shaking, the samples were heated to get a homogenous consistency. A similar protocol was followed for 25 mg PAC and 10 mg alum, 35 mg PAC and 15 mg alum, 45 mg PAC, and 20 mg alum doses.
5% Cation solution used in combination with 1% bleaching powder
Different doses were prepared to use both cation and bleaching powder in combination. 15 mg of cation and 5 mg of bleaching powder were dissolved in 1000 mL water and agitated on a shaker at medium speed. A similar protocol was followed for 25 mg cation and 10 mg bleaching powder, 35 mg cation and 15 mg bleaching powder, 45 mg cation, and 20 mg bleaching powder doses.
5% Ferric chloride solution used in combination with 5% alum
Different doses were prepared to use both ferric chloride solution and alum in combination. 15 mg of ferric chloride solution and 5 mg of alum were dissolved in 1000 mL water and agitated on a shaker at medium speed. A similar protocol was followed for 25 mg ferric chloride and 10 mg alum, 35 mg ferric chloride, 15 mg alum, 45 mg ferric chloride, and 20 mg alum doses.
Waste water Treatment
After shaking, the samples were heated to get a homogenous consistency. The treated samples were allowed to cool down and settle down. The solution was filtered and sludge was discarded. The Filtrate was used to calculate the reduction percentages of different physicochemical parameters and microbial load.
RESULTS
Physiochemical characterization of samples
Physiochemical characterizations (temperature, pH, COD, BOD, and turbidity) of collected samples were analyzed. The temperature was recorded as 17-22℃ in all three samples. The observed pH of waste water collected from all three industries was 6. The recorded COD value of sample KGI was 400 mg/L whereas the COD of the other two samples i.e., HVO and MCO was 300 and 280 mg/L respectively.
The BOD value observed for sample KGI was 360 mg/L whereas the BOD of the other two samples i.e., HVO and MCO was 200 and 210 mg/L respectively. The turbidity of all samples was determined by using a turbidity meter. The highest value i.e., 101 NTU of turbidity was recorded for sample KGI, whereas the turbidity of the other two samples i.e., HVO and MCO were 93 and 88 NTU respectively as indicated in Table 1.
Table 1: Physiochemical characteristics (pH, COD, BOD, and turbidity) of samples
|
Sr. No. |
Sample ID |
Temperature (℃) |
pH |
COD (mg/L) |
BOD (mg/L) |
Turbidity (NTU) |
|
1 |
KGI |
17-22 |
6 |
400 |
360 |
101 |
|
2 |
HVO |
17-22 |
6 |
300 |
200 |
93 |
|
3 |
MCO |
17-22 |
6 |
280 |
210 |
88 |
Microbial determination
Total plate count (TPC), total coliform, and E. coli
After 24-48 h of incubation, TPC/ml was calculated. KGI and HVO samples showed the highest TPCs/ml. MCO water sample showed fewer TPCs/ml with the recorded value of 1.00 x 103 TPCs/ml as shown in Table 2. However, the highest coliform and E.coli count was recorded for KGI followed by MCO and HVO (Table 2).
Table 2. TPC, TCC, and E. coli count of waste water samples
|
Sr. No. |
Samples |
Total Plate Count (CFU/mL) |
Total Coliforms (CFU/mL) |
E. coli (CFU/mL) |
|
1 |
KGI |
TNTC |
7.2 x 102 |
6.2 x 102 |
|
2 |
HVO |
TNTC |
4.4 x 102 |
3.4 x 102 |
|
3 |
MCO |
1.00 x 103 |
5.0 x 102 |
4.0 x 102 |
Treatment with banana and orange peel coagulant
It was observed that banana and orange peels caused a significant reduction in BOD at different pH with different concentrations. Maximum reduction was recorded with 45 mg at pH 6 and minimum reduction was recorded with 15 mg at pH 5 and 8. Reduction in COD was recorded at different pHs. With 45 mg coagulant, the maximum reduction was recorded at pH 6 and minimum reduction was recorded with 15 mg at pH 5. The recorded values showed that banana and orange peels caused a significant reduction in turbidity with different concentrations at different pH. Maximum reduction in turbidity was recorded with 45 mg at pH 6 and minimum reduction was recorded with 15 mg at pH 5 and 8. Significant reduction in E. coli at different pH with different concentrations was observed with banana and orange peels. Maximum reduction in E. coli was recorded with 45 mg at pH 6 and 8 and minimum reduction was recorded with 45 mg at pH 5 as shown in Fig. 1.
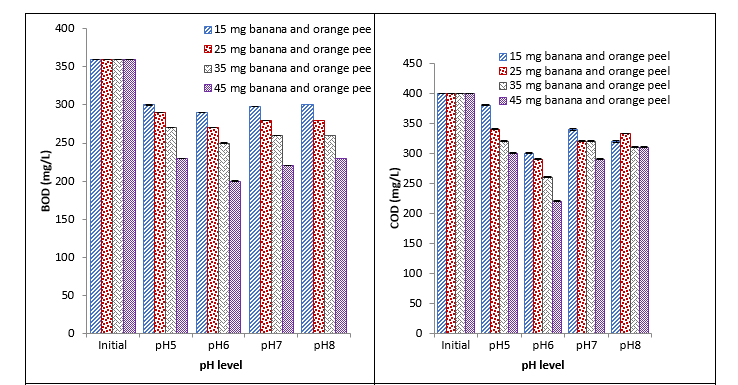
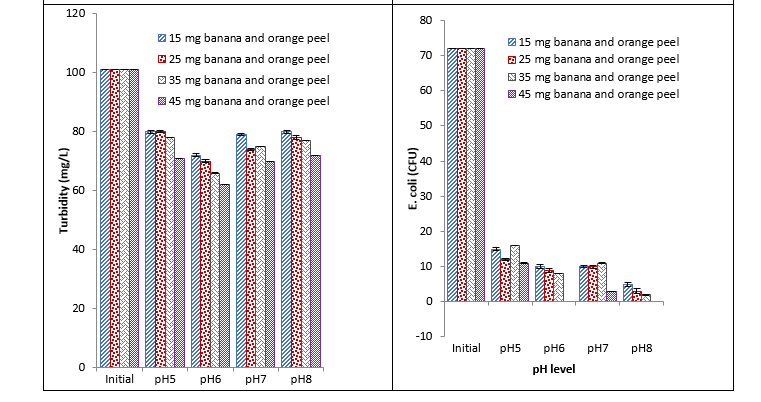
Fig. 1. Effect of different concentrations of banana and orange peel coagulant on BOD, COD, turbidity, and E. coli at different pH
Treatment with date seeds coagulant
It was observed that date seeds caused a significant reduction in BOD at different pH with different concentrations. With an increase in concentration, a continuous decrease in BOD was recorded. Maximum reduction was recorded with 45 mg at pH 6, and minimum reduction was recorded with 15 mg at pH 5, 7, and 8. Similar results were recorded for COD, turbidity, and E. coli as an increase in the concentration of coagulant results in a continuous decrease. Moreover, a maximum reduction in COD, turbidity, and E. coli was recorded at 45 mg at pH 6, and a minimum reduction was recorded at 15 mg at pH 5. The recorded values showed that date seeds caused a significant reduction in COD, turbidity, and E. coli with different concentrations at different pH as indicated in Fig. 2.
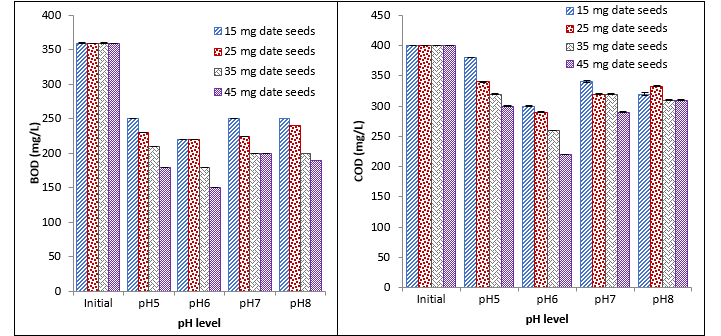
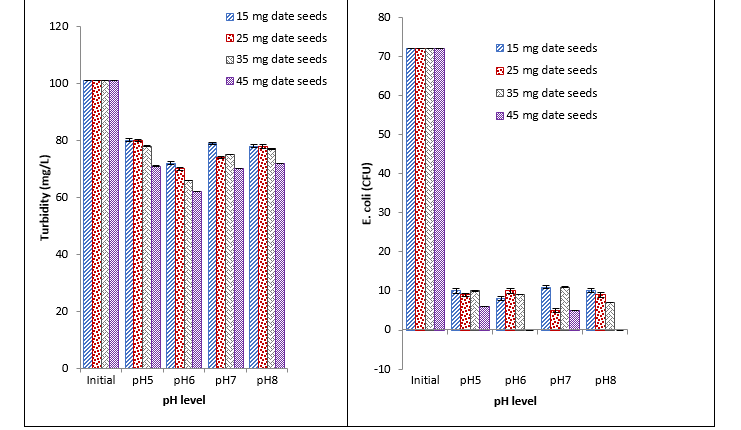
Fig. 2. Effect of different concentrations of date seeds coagulant on BOD, COD, turbidity, and E. coli at different pH
Treatment with PAC and alum
It was observed that PAC and alum caused a significant reduction in different physicochemical parameters and E. coli at different pH with different concentrations. With an increase in the concentration of coagulants, a decrease in different parameters was recorded. Maximum reduction was recorded with 45 mg at pH 6 and minimum reduction was recorded with 15 mg at pH 7 as shown in Fig. 3.
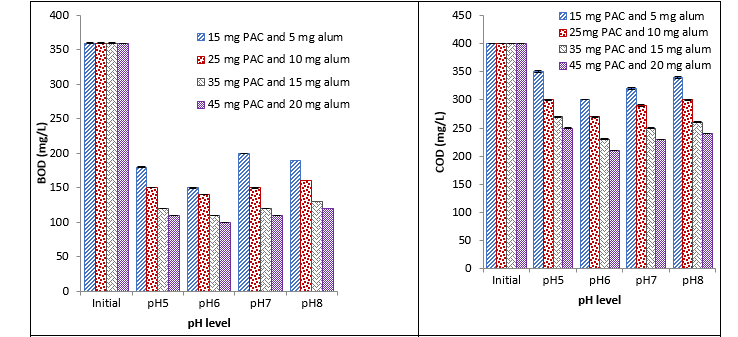
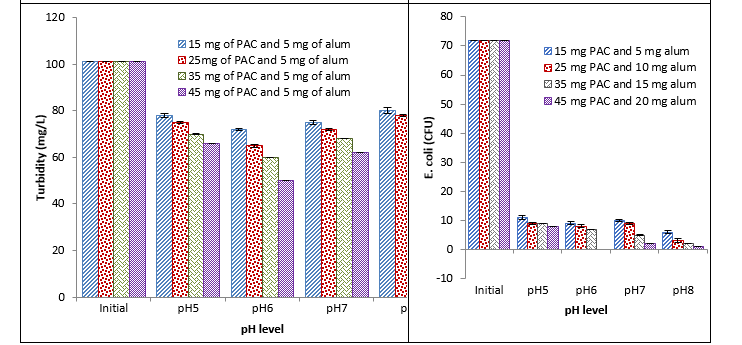
Fig. 3. Effect of different conc. of PAC and alum coagulant on BOD, COD, turbidity, and E. coli at different pH
Treatment with Ca+2 cation and bleaching powder
It was observed that Ca+2 cation and bleaching powder caused a significant reduction in all the observed parameters at different pH with different concentrations. With the increase in concentration continuous decrease was recorded in all studied parameters. Maximum reduction was recorded with 45 mg at pH 6 and minimum reduction was recorded with 15 mg at pH 5 as shown in Fig. 4.

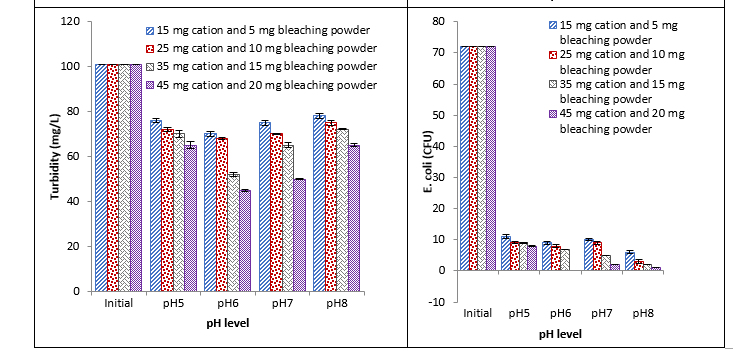
Fig. 4. Effect of different concentrations of Ca+2 cations and bleaching powder coagulant on BOD, COD, turbidity, and E. coli at different pH
Treatment with ferric chloride and alum
The diverse and proficient results were observed with ferric chloride and alum combination. Significant reduction in BOD, COD, and turbidity at different pH with different concentrations was observed. Maximum reduction with all studied parameters such as BOD, COD, turbidity, and E. coli was recorded with 45 mg at pH 6 and minimum reduction was recorded with 15 mg at pH 8, pH 5, pH 5, and pH 8 respectively. It was observed that ferric chloride and alum cause significant reduction in E. coli at different pH with different concentrations. With an increase in concentration, a continuous decrease was recorded. Maximum reduction was recorded with 45 mg at pH 6 and little reduction was recorded with 45 mg at pH 5 as shown in Fig. 5.
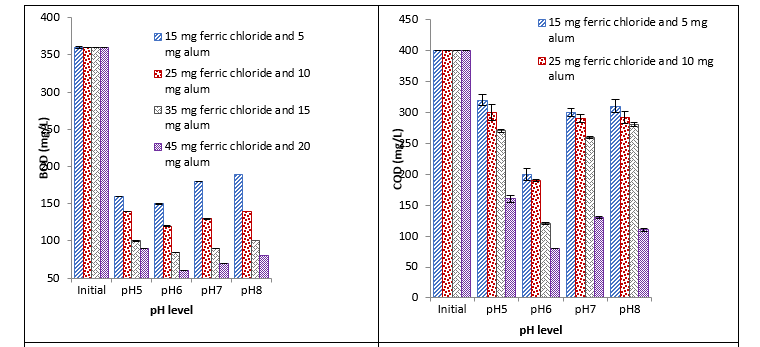
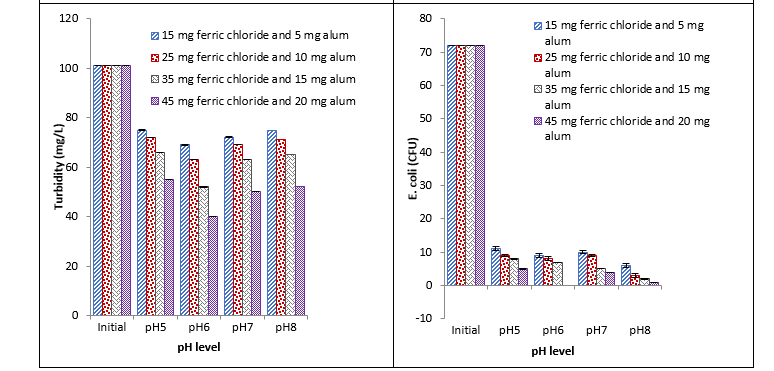
Fig. 5. Effect of different concentrations of ferric chloride and alum coagulant on BOD, COD, turbidity, and E. coli at different pH
DISCUSSION
Water resources are becoming inadequate in their clean state because of industrial advancements in recent years. Water pollution harms human health and the environment [15]. Waste water of the food industry comprises numerous pollutants and contaminants that are taking an equal role in raising ecological issues [16].
In developing countries, including Pakistan, waste water is not properly treated and is usually discarded without treatment [17]. This untreated waste water discharge is the cause of many diseases directly either by mixing in water bodies or indirectly by irrigating crops. For water treatment, chemical coagulants are used. Harmful effects and sludge production in large quantities by using chemical coagulants increase the need for alternatives. Natural coagulants are being used to treat waste water and improve water quantity. The major aim of the present work was to evaluate the microbial load in wastewater from oil industries and waste water treatment using coagulants.
The waste water samples were collected from Khalis Group of Industries, Hamza Vegetable Oil Refinery, Ghee Mills, and Momin Cooking Oil and Ghee Mills Lahore. All the significant parameters like COD, BOD, turbidity, pH, and E. coli were measured.
The impact and influence of all these parameters differ in different samples of wastewater because of a load of biological and non-biological content in polluted water. The present study has been based on the effectiveness of some of the commonly used coagulating agents. These coagulating agents were used to treat the polluted wastewater released from the edible oil industry. The unstable experimental situation evaluated the industrial effluents. Various thickening agents conducted the practical work. However, some of them were discarded at the initial stage because of less productivity and inefficiency.
The impact of the dose of coagulants, pH, and composition of the coagulating agents was investigated to evaluate the experimental methodology's proficiency. Residual agricultural waste especially seeds, fruit shells, and peels are significant to be used as adsorbents to treat wastewater. The usage of agricultural residual to treat waste water not only enhances the value of residual waste but also reduces the growing problem to treat this residual waste [18, 19].
It was observed that parameters like COD, BOD, turbidity, and pH describing the quality of water have values significantly higher as compared to National Standards for Drinking Water Quality commonly known NEQS standards [20]. COD, BOD, and turbidity were higher in sample KGI. TVC, TCC, and E. coli were also higher in KGI as compared to HVO and MCO which indicates that excessive presence of BOD in waste water has strongly influenced the physicochemical properties of water given by the World Health Organization (WHO) and Environmental Protection Agency (EPA) [21].
It was observed that 15mg banana and orange peel showed a high reduction in BOD at pH 8. One of the reasons for the higher reduction of BOD at such a low concentration of natural coagulant is might be due to the presence of nitrogen, sulfur atoms, and carboxylic acids like compounds in banana peel, and similarly orange peels are also rich in citric acid. A total of 45mg banana and orange peels coagulant showed maximum reduction in BOD and COD at pH 8. Nearly 15 mg and 25 mg of banana and orange peels coagulant reduced maximum COD at pH 5 respectively. Turbidity was decreased by 45 mg banana and orange peel coagulant at pH 8, while a maximum reduction in E. coli (CFU) was observed at pH 5. The results were in concordance with the work of Beatrice and co-researchers who reported that banana peels can be used as a substitute for chemical coagulant as it reduces the BOD, COD, turbidity, and many metals in waste water. However, it increases nitrites, iron, and manganese. Hence, additional treatment is required [22]. In another study, an effective result was recorded at pH 4 with banana and orange peel against leather effluents [23]. However, in one more study best results with banana and orange peel were found at pH 6-8 [24] and similar findings were noticed in our study.
Date seeds have greater potential to work as antioxidants, anti-viral and anti-bacterial agents. It was observed that 45 mg date seed showed a high reduction in BOD and COD at pH 7. Total 15 mg date seed coagulant reduced maximum COD at pH 5. Turbidity was decreased by 45 mg date seed coagulant at pH 8. While a maximum reduction in E. coli (CFU) was observed at pH 5. The possible reason that E. coli number is found reduced with natural coagulants at low pH such as 4 and 5 is due to acidic conditions of the media that restrict the growth of E. coli, the normal survival of this species is good at pH 7 and 8. Another study indicated that dates removed 75% turbidity, 78% COD, 85% BOD, and 88% total coliform in wastewater. The removal efficiency of dates is slightly lower as compared to other coagulants [25]. In various studies date seeds that were chemically modified as nano-particle or simply treated with chemicals showed promising results against waste water [26, 27].
The experimental work with natural coagulants indicates that the removal rate of COD, BOD, turbidity, and E. coli has been increased with the increased dosage of coagulants and produced eco-friendly effects. At lower rate efficiency is less however, efficiency improves by surging the dose of coagulants. It is previously known that the removal efficiencies of different natural coagulants are different. Therefore, the selection of coagulants is important for efficient treatment [28].
It was observed that all the chemical coagulants (PAC with alum, Ca+2 cation with bleaching powder, ferric chloride with Alum) reduced the physicochemical indicators and microbial load when used in small concentrations at different pH.
It was observed that a little quantity of chemical coagulant is sufficient for water treatment. While a large quantity or high dose of natural coagulant is required to treat wastewater. Moreover, natural coagulants showed an irregular pattern of efficiency when used to reduce BOD, COD, and turbidity. This study indicated that natural coagulants are less effective to improve the quality of water. While chemical coagulants raised health and environmental issues so there is a need to go with environment-friendly strategies [29].
CONCLUSION
In developing countries like Pakistan, waste water is discarded without treatment which increases the risk of diseases by many folds. For the waste water treatment, chemical and natural coagulants were used. It was concluded that chemical coagulants were more efficient to treat waste water than natural coagulants. However, a detailed study is required to select a more appropriate and to find a natural coagulant. As natural coagulants are environment friendly and better remedies to adopt.
REFERENCES
[1] Edokpayi, J. N., Odiyo, J. O., & Durowoju, O. S., “Impact of wastewater on surface water quality in developing countries: a case study of South Africa” 2017. Water quality, 10: 66561.
[2] Owa, F. D., “Water pollution: sources, effects, control, and management” 2013. Mediterranean journal of social sciences, Vol 4, Issue 8, pp 65-65.
[3] Sharma, P., Tripathi, S., & Chandra, R., “Highly efficient phytoremediation potential of metal and metalloids from the pulp paper industry waste employing Eclipta alba (L) and Alternanthera philoxeroide (L): Biosorption and pollution reduction” 2021. Bioresource Technology, Vol 319, Issue 1, pp 124-147.
[4] Ado, A., Tukur, A. I., Ladan, M., Gumel, S. M., Muhammad, A. A., Habibu, S., & Koki, I. B., “A review on industrial effluents as major sources of water pollution in Nigeria, 2015. Chemistry Journal, Vol 1, Issue 5, pp 159-164.
[5] Galadima, A., Garba, Z. N., Leke, L., Almustapha, M. N., & Adam, I. K., “Domestic water pollution among local communities in Nigeria-causes and consequences” 2011. European journal of scientific research, Vol 52, Issue 4 pp 592-603.
[6] Aniyikaiye, T. E., Oluseyi, T., Odiyo, J. O., & Edokpayi, J. N., “Physico-chemical analysis of wastewater discharge from selected paint industries in Lagos, Nigeria” 2019. International journal of environmental research and public health, Vol 16, Issue 7, pp 12-35.
[7] Boelee, E., Geerling, G., van der Zaan, B., Blauw, A., & Vethaak, A. D., “Water and health: From environmental pressures to integrated responses” 2019. Acta tropica, Vol 19, Issue 31, pp 217-226.
[8] Ang, W.L. and A.W. Mohammad, “State of the art and sustainability of natural coagulants in water and wastewater treatment” 2020. Journal of Cleaner production, Vol 262, Issue 1, pp121267.
[9] Gandiwa, B. I., Moyo, L. B., Ncube, S., Mamvura, T. A., Mguni, L. L., & Hlabangana, N., “Optimisation of using a blend of plant based natural and synthetic coagulants for water treatment: (Moringa Oleifera-Cactus Opuntia-alum blend)” 2020. South African Journal of Chemical Engineering, Vol 34, Issue 1, pp 158-164.
[10] Gautam, S. and G. Saini., “Use of natural coagulants for industrial wastewater treatment” 2020. Global Journal of Environmental Science and Management, Vol 6, Issue 4, pp553-578.
[11] Ilyas, M., Ahmad, W., Khan, H., Yousaf, S., Yasir, M., & Khan, A., “Environmental and health impacts of industrial wastewater effluents in Pakistan: a review” 2019. Reviews on environmental health, Vol 34, Issue 2, PP171-186.
[12] Walter, W.G., “Standard methods for the examination of water and wastewater”, 1961, American Public Health Association.
https://engage.awwa.org/PersonifyEbusiness/Store/Product-Details/productId/65266295
[13] Ekopai, J. M., Musisi, N. L., Onyuth, H., Gabriela Namara, B., & Sente, C., “Determination of bacterial quality of water in randomly selected swimming pools in Kampala City, Uganda” 2017. New Journal of Science, Vol 4, Issue 1, pp1-7.
[14] An, Y.-J., D.H. Kampbell, & G.P., “Breidenbach, Escherichia coli and total coliforms in water and sediments at lake marinas” 2002. Environmental Pollution, Vol 120, Issue 3, pp 771-778.
[15] Haseena, M., Malik, M. F., Javed, A., Arshad, S., Asif, N., Zulfiqar, S., & Hanif, J., “Water pollution and human health” 2017. Environmental Risk Assessment and Remediation, Vol 1, Issue 3, pp 1-15.
[16] Cristian, O., “Characteristics of the untreated wastewater produced by food industry”, 2017. Analele Universităţii din Oradea, Fascicula: Protecţia Mediului, Vol 15, Issue 1, pp 709-714.
[17] Boutilier, L., Jamieson, R., Gordon, R., Lake, C., & Hart, W., “Adsorption, sedimentation, and inactivation of E. coli within wastewater treatment wetlands” 2009. Water research, 2009. Vol 43, Issue 17, pp4370-4380.
[18] Murtaza, G. & M.H. Zia., “Wastewater production, treatment and use in Pakistan. in Second regional workshop of the project ‘safe use of wastewater in agriculture”, 2012.
[19] Matilainen, A., M. Vepsäläinen, & M. Sillanpää, “Natural organic matter removal by coagulation during drinking water treatment: A review” 2010. Advances in colloid and interface science, Vol 159, Issue 2, pp189-197.
[20] Imran, H., “Wastewater monitoring of pharmaceutical industry: treatment and reuse options” 2005. Electron J Environ Agric Food Chem, Vol 4, Issue 4, pp 994-1004.
[21] Anjaneyulu, Y., N.S. Chary, & D.S.S. Raj., “Decolourization of industrial effluents–available methods and emerging technologies–a review” 2005. Reviews in Environmental Science and Bio/Technology, Vol 4, Issue 4, pp245-273.
[22] Kakoi, B., Kaluli, J. W., Ndiba, P., & Thiong’o, G., “Banana pith as a natural coagulant for polluted river water” 2016. Ecological engineering, Vol 95, Issue 1, pp699-705.
[23] Temesgen, F., Gabbiye, N., & Sahu, O., “Biosorption of reactive red dye (RRD) on activated surface of banana and orange peels: economical alternative for textile effluent” 2018. Surfaces and interfaces, Vol 12, Issue 1, pp151-159.
[24] Al Khusaibi, T. M., Dumaran, J. J., Devi, M. G., Rao, L. N., & Feroz, S, “Treatment of dairy wastewater using orange and banana peels” 2015. Journal of Chemical and Pharmaceutical Research, Vol 7, Issue 4, pp1385-1391.
[25] Kuhiyop, E., D. Adie, & U. Abubakar, “Application of Mangifera indica (mango) and Phoenix dactylifera (dates) seeds powders as coagulants in wastewater treatment” 2020. Nigerian Journal of Technology, Vol 39, Issue 1, pp269-277.
[26] Rambabu, K., Bharath, G., Banat, F., & Show, P. L., “Green synthesis of zinc oxide nanoparticles using Phoenix dactylifera waste as bioreductant for effective dye degradation and antibacterial performance in wastewater treatment” 2021. Journal of hazardous materials, Vol 402, Issue 1, pp123-560.
[27] Abdus-Salam, N., Ikudayisi-Ugbe, A. V., & Ugbe, F. A., “Adsorptive removal of methylene blue from synthetic wastewater using date palm seeds, goethite and their composite” 2021. Acta Scientifica Malaysia (ASM), Vol 5, Issue 1, pp27-35.
[28] Nimesha, S., Hewawasam, C., Jayasanka, D. J., Murakami, Y., Araki, N., & Maharjan, N., “Effectiveness of natural coagulants in water and wastewater treatment” 2021. Global Journal of Environmental Science and Management. Vol 8, Issue 1,pp101-116.
[29] Kurniawan, S. B., Abdullah, S. R. S., Imron, M. F., Said, N. S. M., Ismail, N. I., Hasan, H. A., Othman, A.R., & Purwanti, I. F, “Challenges and opportunities of biocoagulant/bioflocculant application for drinking water and wastewater treatment and its potential for sludge recovery” (2020). International Journal of Environmental Research and Public Health, Vol 17, Issue 24, 9312.
 |
Copyright © by authors and 50Sea. This work is licensed under Creative Commons Attribution 4.0 International License. |




















