FIRST REPORT OF GENUS PARMELIELLA MÜLL. (PELTIGERALES; LECANOROMYCETES; ASCOMYCOTA) FROM PAKISTAN
Qudsia Firdous1, Arslan Ali2, Abdul Nasir Khalid1
1Institute of Botany, University of the Punjab, Lahore 54590, Pakistan.
2International Center for Chemical and Biological Sciences, University of Karachi, University Road, Karachi-75270, Pakistan.
* Correspondence: Qudsia Firdous qudsiafirdous26@gmail.com
Citation | Firdous. Q, Ali. A, Khalid. A. N, “First Report of Genus PARMELIELLA MÜLL. (Peltigerales; Lecanoromycetes; Ascomycota) From Pakistan” International Journal of Innovations in Science and Technology, Vol 4, Issue 2, 2022, pp: 450-458
Received | April 28, 2022; Revised | May 12, 2022; Accepted |May 16, 2022; Published | May 18, 2022.
When studying lichens in Pakistan, we came across a crustose species with small to moderate squamulose on a thin blackish hypothallus with a dry, rough, gray-brown to the black upper surface. The standard chemical tests integrated with conventional to modern taxonomic tools were used to name the specimen. Consequently, with minor differences in the morphology, and no difference in nucleotides, the lichen species was baptized Parmeliella thriptophylla (Ach.) Müll. Arg. The descriptive taxonomy and n-ITS-based phylogeny of this species with its habitus are presented in this study. No previous record of this species, genus, or family was found in Pakistan.
Keywords: Parmeliella thriptophylla; phylogeny; geography; Azad Jammu & Kashmir; ITS rDNA
INTRODUCTION
Parmeliella Müll. is categorized as a lichen genus having crustose-squamulose to foliose thallus spread on a cottony prothallus, biatorine apothecia, with or without thalline margins; hymenium I+ persistent blue; asci with an apical amyloid plug that is unique and hyaline and simple ascospores [1]. It has three forms; Parmeliella Müll. Arg. 1862, Parmeliella sect. Austroparmeliella P.M. Jørg. 2004 and Parmeliella sect. Parmeliella Müll. Arg. 1862 (http://www.indexfungorum.org/Names/Names.asp). A recent estimate places 100 species in this genus [2]. It belongs to the family Pannariaceae, order Peltigerales [3]. The family has a widespread distribution, but its species are especially ubiquitous in southern temperate regions [4]. Wedin & Wiklund, (2004)[5] treated this family under the monophyletic suborder Peltigerineae having approximately 27 genera reported from different parts of the world. From Pakistan, we found P. thriptophylla (Ach.) Müll. Arg., also known as Parmeliella triptophylla (Ach.)[6], is a basionym of Lecidea thriptophylla Ach. 1808, representing the first record for the country. The study illustrates morpho-anatomical diagnostic characters and molecular confirmations in the phylogenetic tree. Comparisons with previous descriptions and data on their distributions are briefly discussed. It is observed that this species has a minimal distribution range and has disjunctive dissemination.
Material and Methods.
Collection site and morphology
The sample for this study was collected during a lichen survey of different sites of Azad Jammu and Kashmir, Pakistan, in 2018 (Figure 2.). Morphological characters were observed under a stereomicroscope (Meiji Techno, EMZ-5TR, Japan). Standard microscopy and spot tests [7] were used for further identification. Measurements were made from freehand sections of thallus mounted in water on glass slides. The microscopic features were observed under a compound microscope (MX4300H, Meiji Techno, Japan).
DNA extraction and PCR amplification
DNA from the thallus was extracted using a 2% CTAB protocol [7]. Molecular data was generated for the internal transcribed spacer (ITS) region. The primer pair ITS1F [8] and ITS4 was used to amplify the ITS region under the PCR conditions used by Khan et al. (2018)[9]. PCR products were visualized in a 1% agarose gel [10] and sent to BGI Hong Kong for sequencing.
Phylogenetic analysis
The ITS locus was amplified and sequenced for the lichen specimen. The BioEdit sequence alignment editor was used to reassemble forward and reverse sequences [11]. The nucleotide sequence comparison was performed using the Basic Local Alignment Search Tool (BLAST) of the National Centre for Biotechnology Information (NCBI) [12]. The closely matching sequences were downloaded from GenBank for subsequent phylogenetic analysis (Table 1). Multiple sequence alignment was performed using MAFFT v 7.0 with all parameters set to default values [13]. The aligned sequences were trimmed from both 5' and 3' ends at conserved sites. Maximum Likelihood analysis was performed using the software MEGA v 7.0 [14]. One thousand rapid bootstrap replicates were run to infer the evolutionary history of the species using the Kimura 3-parameter model. The length of the final aligned file was 1304 nucleotides, of which 296 sites were conserved, 418 variables, 203 parsimony-informative and 141 were singletons. Degelia plumbea (Lightf.) P.M. Jørg. & P. James (AF429265) was chosen as an outgroup.
Results and discussion.
Parmeliella thriptophylla was growing on exposed sedimentary rocks and soil in moist and shady sites in a mountainous landscape characterized by fertile, green, rocky, and undulating territory, Azad Jammu and Kashmir [15]. The taxon is maybe not the easiest to recognize due to its appearance and pattern of distribution. It has small, brown to blue-grey squamules [16]. We also described morphological descriptions accompanied by colored photographs of the thallus and microscopic structures (Table 3). In comparison, a few morphological differences were observed (Plate 1). The color of thallus from America is blue-brown [17]; from Norway, it is reported as dark blue-black while brown-black in our findings. Lobes and lobules were also seen in previous studies from Europe and the South of Nordland [13]; our specimen was non-lobulated. It is also different in having non-branched isidia in our case vs. branched isidiate from the previously reported species [14]. Except for a bit of difference in the sizes of macroscopic and microscopic structures, it was more or less similar to other world collections. The similar features, squamulose thallus, granular and marginal isidia, absence of lower cortex, and negative spot tests revealed the taxon identity. Phylogenetically, our lichen collection (MW255137) was 100% matched with P.thriptophylla, reported from the different parts of the world (Table 1). Our sequence clustered in the tree with accession numbers HM448804 and KC618727 submitted from Sweden (Figure 1). It showed robust bootstrap (100%) value for the phylogenetic support. P. nigrocincta (KC618724) and P. thysanot (KC618726) made sister clades to the P.thriptophylla. This fork-shaped structure is further divided to form another clade with different lengths of branches for each specific species. The first clade is further extended for P. parvula and then developed with two sister clades containing other species. The branching pattern in a phylogenetic tree reflects ancestor-descendant relationships, having more common ancestors. Black nodes represent our node species. The geographical comparison showed the species had been reported from temperate and tropical areas, primarily in cool-temperate, widespread in the Northern Hemisphere (Table 2)
It is not known with certainty from the African continent; however, present in Macaronesia [10], [14], Andes and Central America [14] and Russia [18], Kodagu district and India [19] for which detail taxonomic descriptions are not available. However, in this study, we describe the species for the first time from the warm and temperate areas of Sharda Valley, Azad Jammu, and Kashmir, Pakistan, as a first record.
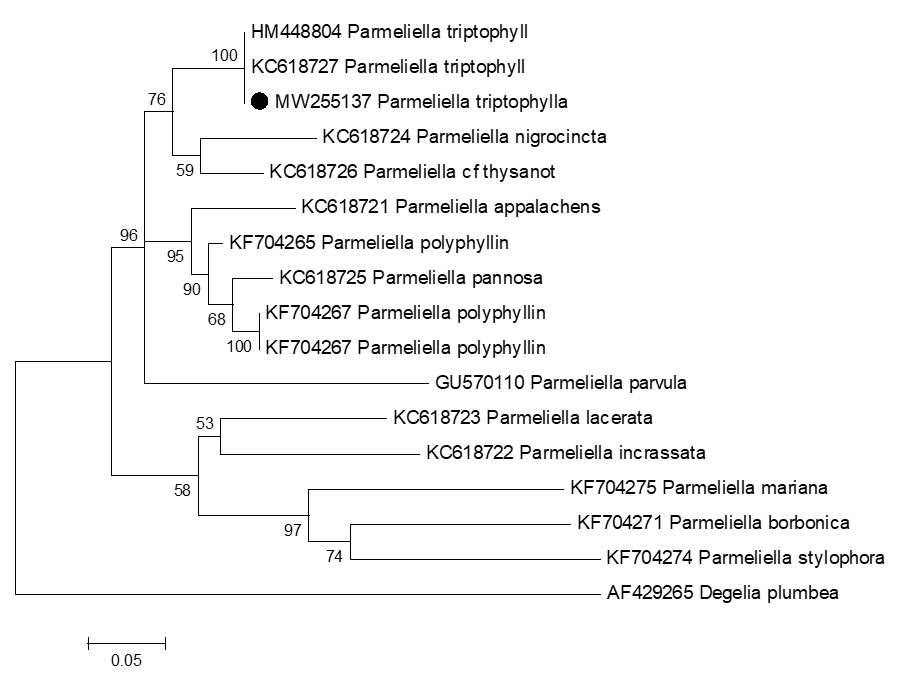
Figure 1. Phylogenetic analysis of Parmeliella thriptophylla species based on nrITS–rDNA regions. This tree is based on maximum likelihood method using Tamura 3-parameter model. The bootstrap values are given above branches. Species collected from Pakistan have been labeled with a box ( ).
Table 1. Parmeliella species with their accession numbers of ITS sequences used in this study
|
Accession Numbers |
Species Names |
Country |
References |
|
MW255137 |
Parmeliella thriptophylla |
Pakistan |
Current Study |
|
HM448804 |
Parmeliella thriptophylla |
Sweden |
Unpublished |
|
KC618727 |
Parmeliella thriptophylla |
Sweden | Unpublished |
|
KC618726 |
Parmeliella cf thysanota |
Sweden |
Unpublished |
|
KF704265 |
Parmeliella polyphyllina |
Belgium |
Magain & Sérusiaux 2014 |
|
KC618724 |
Parmeliella nigrocincta |
Sweden |
Unpublished |
|
KF704267 |
Parmeliella polyphyllina |
Belgium |
Magain & Sérusiaux 2014 |
|
KC618721 |
Parmeliella appalachensis |
Sweden |
Unpublished |
|
KC618725 |
Parmeliella pannosa |
Sweden |
Unpublished |
|
KC618723 |
Parmeliella lacerate |
Sweden |
Unpublished |
|
KC618722 |
Parmeliella incrassate |
Sweden |
Unpublished |
|
KF704267 |
Parmeliella polyphyllina |
Belgium |
Magain & Sérusiaux 2014 |
|
KF704271 |
Parmeliella borbonica |
Belgium |
Magain & Sérusiaux 2014 |
| GU570110 |
Parmeliella parvula |
Norway |
Unpublished |
| KF704275 |
Parmeliella mariana |
Belgium |
Magain & Sérusiaux 2014 |
|
KF704274 |
Parmeliella stylophora |
Belgium |
Magain & Sérusiaux 2014 |
|
AF429265 |
Degelia plumbea |
Norway |
Ekman & Jørgensen 2002 |
Table 2. ITS sequences present in Genebank for Parmeliella thriptophylla overall
|
Countries |
Accession Numbers |
Geography of the areas | Climatic Conditions |
|
Sweden |
HM448804 KC618727 |
Parmeliella thriptophylla Parmeliella thriptophylla voucher Wedin 7037 (UPS) | Temperate climate |
| South America | MH802366MH802347 MH887519 | Parmeliella thriptophylla voucher NK-278 Parmeliella thriptophylla voucher NK-278 | Tropical rain forest |
| Alaska, USA | MN437620 MN508285MN483131MN483097 MN483096 MN460220 | Parmeliella thriptophylla voucher Spribille | Frigid winters and short, cool summers |
| Norway | AF429269MK812457MH802418 | Parmeliella thriptophylla voucher Ekman 3203 (BG) Parmeliella thriptophylla voucher O-L--207999 Parmeliella thriptophylla voucher NK-278 | Marine climate, with comparatively cool summers, mild winters |
Table 3. Morphological notes of Parmeliella thriptophylla (Ach.) Müll.Arg. from Pakistan
|
Lichen Features |
Description |
|
Morphological features of thallus
|
Small to moderate size squamulose, resting on a thin blackish hypothallus that contributes to the dark color of this species; Squamules: up to 1 mm wide, incised; Upper surface: dry, rough, gray-brown to black, isidiate but not sorediate; Isidia: coralloid, digitate to granular mostly, marginal, sometimes obscuring the squamules; Pycnidia: not seen; Apothecia: Not present |
|
Thallus section |
Thick upper cortical layer present, 16-18 um high, hyaline; Cortical cells: two rows of cells can be seen easily, square to irregular to multiangular, 3-6 um long |
|
Photobiont layer |
Yellowish green, very thick, 45-55 um high; Lower layer: thin, darker (blackish), only up to 9 to 11 um high |
|
Spot test |
Cortex: K-, C-, KC- |
|
Substrate and Ecology |
On rocks in a Himalayan moist temperate forest, at an altitude of 1,943 m.a.s.l., shaded, maximum and minimum temperature of 28oC and -2 oC, respectively, annual rainfall varying between 800–1200 mm. |
|
Material examined |
PAKISTAN. Azad Jammu & Kashmir: Neelam Valley, Sharda; 34.7931° N, 74.1930° E; July 25, 2019; A. N. Khalid and Q. Firdous. KSH-13, LAH36782 |

Plate 1. Parmeliella thriptophylla A: Thallus morphology along with the substrate B & C: Sections of thallus
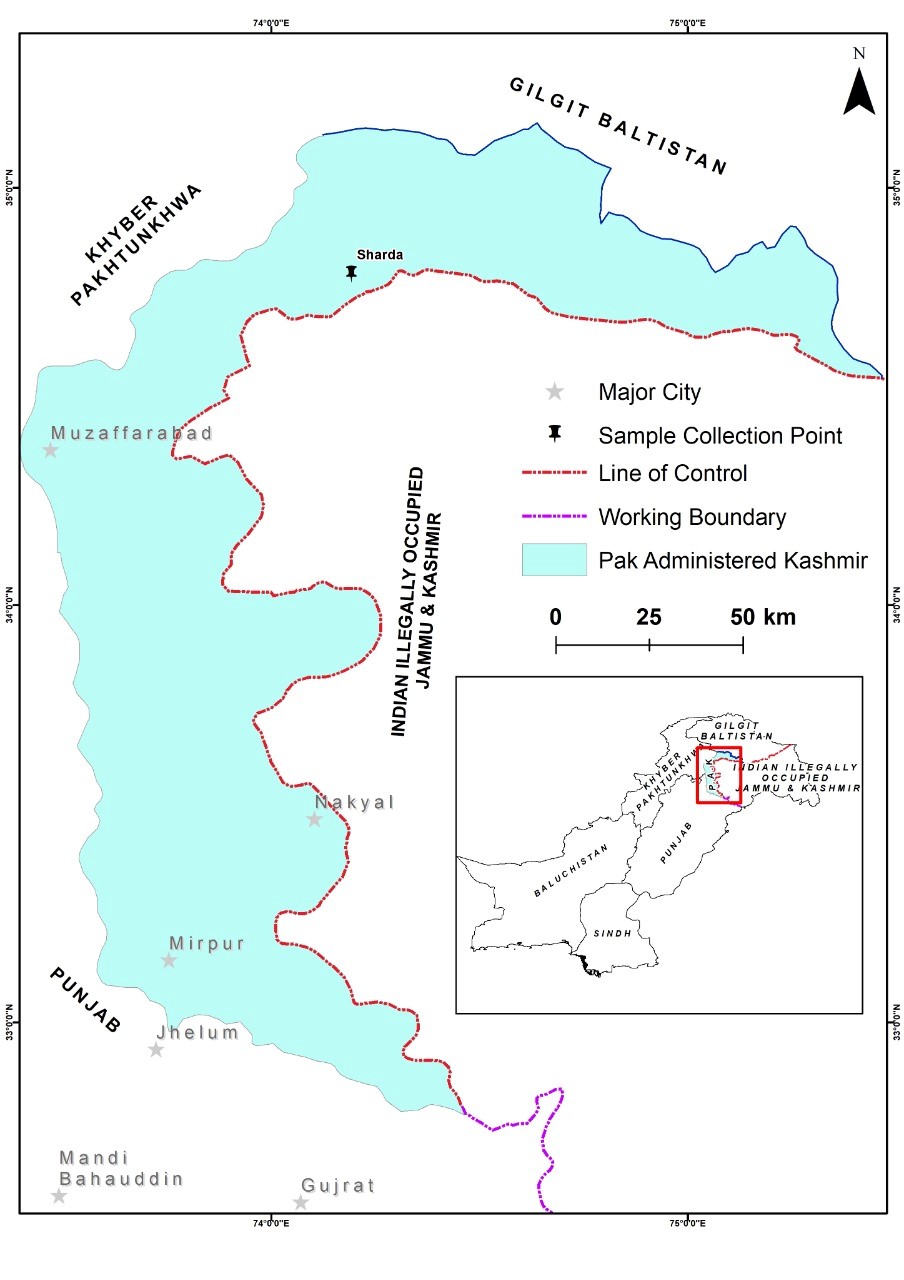
Figure 2. Map of the sampling site
CONCLUSION
In this study, we present the molecular taxonomy of Parmeliella thriptophylla and its family for the first time from Pakistan. The detailed macroscopy and microscopy of the species strongly supported its classification and proof of phylogeny. Here, we mentioned the geographical positions and compared the climatic conditions to see their effect on the specimen's morphology. It has been established that an area's physical conditions influence the outlook of living organisms but have little or no effect on the nucleotide of the specimen.
REFERENCES
[1] P. M. Jørgensen, “NEW OR INTERESTING PARMELIELLA SPECIES FROM THE ANDES AND CENTRAL AMERICA,” Lichenol., vol. 32, no. 2, pp. 139–147, Mar. 2000, doi: 10.1006/LICH.1999.0259.
[2] P. M. Jørgensen, “Notes on Some Misunderstood, Subtropical Parmeliella Species on JSTOR,” Bryologist, vol. 106, no. 1, pp. 121–129, 2003.
[3] S. Ekman, M. Wedin, L. Lindblom, and P. M. Jorgensen, “Extended phylogeny and a revised generic classification of the Pannariaceae (Peltigerales, Ascomycota),” Lichenol., vol. 46, no. 5, pp. 627–656, 2014, doi: 10.1017/S002428291400019X.
[4] P. F. Cannon and P. F. Kirk, “Fungal families of the world,” Fungal Fam. world, 2007, doi: 10.1079/9780851998275.0000.
[5] E. Wiklund and M. Wedin, “The phylogenetic relationships of the cyanobacterial lichens in the Lecanorales suborder Peltigerineae,” Cladistics, vol. 19, no. 5, pp. 419–431, 2003, doi: 10.1111/j.1096-0031.2003.tb00312.x.
[6] B. P. Lofall, “Stiftfiltlav Parmeliella triptophylla i Østfold,” Natur i Østfold, INNHOLD, pp. 136–138, 1995.
[7] T. D. BRUNS and M. GARDES, “Molecular tools for the identification of ectomycorrhizal fungi--taxon-specific oligonucleotide probes for suilloid fungi,” Mol. Ecol., vol. 2, no. 4, pp. 233–242, 1993, doi: 10.1111/J.1365-294X.1993.TB00013.X.
[8] S. Ekman and P. M. Jørgensen, “Towards a molecular phylogeny for the lichen family Pannariaceae (Lecanorales, Ascomycota),” Can. J. Bot., vol. 80, no. 6, pp. 625–634, 2002, doi: 10.1139/B02-043.
[9] M. Khan, A. N. Khalid, and H. Thorsten Lumbsch, “A new species of Lecidea (Lecanorales, Ascomycota) from Pakistan,” MycoKeys, vol. 38, no. 38, p. 25, Aug. 2018, doi: 10.3897/MYCOKEYS.38.26960.
[10] J. Hafellner, “Towards a better circumscription of the Acarosporaceae ( lichenized CryptogamicBotany,” no. January 1995, 2016.
[11] M. E. Hale, “How to know the lichens,” p. 226, 1969.
[12] “BIOEDIT: A USER-FRIENDLY BIOLOGICAL SEQUENCE ALIGNMENT EDITOR AND ANALYSIS PROGRAM FOR WINDOWS 95/98/ NT | Semantic Scholar.” .
[13] D. L. Hawksworth, “The Lichen Family Pannariaceae in Europe. By Per M. Jørgensen. [Opera Botanica No. 45.] Swedish Natural Science Research Council, Stockholm. 20 November 1978. Pp. 123, figures 53, tables 8. Price SKr 110 (SKr 66 for personal use).,” Lichenol., vol. 11, no. 2, pp. 204–204, Jun. 1979, doi: 10.1017/S0024282979000244.
[14] P. M. Jørgensen, “New or Interesting Parmeliella species from the andes and central America,” Lichenol., vol. 32, no. 2, pp. 139–147, 2000, doi: 10.1006/LICH.1999.0259.
[15] K. Habib and A. N. Khalid, “New records of lichens from the State of Azad Jammu and Kashmir, Pakistan corroborated by ITS sequences,” Nov. Hedwigia, vol. 109, no. 3–4, pp. 457–473, 2019, doi: 10.1127/nova_hedwigia/2019/0552.
[16] T. H. I. Nash, B. D. Ryan, P. Diederich, C. Gries, and F. Bungartz, “Lichen flora of the greater Sonoran Desert region, vol. 1,” vol. 1, 2002, Accessed: May 12, 2022. [Online]. Available: https://heritage.nv.gov/documents/lichen-flora-of-the-greater-sonoran-desert-region-vol-1.
[17] T. H. Nash, Lichen flora of the greater Sonoran Desert region. Tempe Ariz.: Lichens Unlimited Arizona State University, 2002.
[18] G. Urbanavichus and A. Ismailov, “New records of lichens and lichenicolous fungi from Dagestan, Russia,” Folia Cryptogam. Est., vol. 53, no. January, pp. 65–69, 2016, doi: 10.12697/fce.2016.53.08.
[19] S. Rashmi and H. G. Rajkumar, “Diversity of Lichens along Elevational Gradients in Forest Ranges of Chamarajanagar District, Karnataka State,” Int. J. Sci. Res. Biol. Sci., vol. 6, no. 1, pp. 97–104, Feb. 2019, doi: 10.26438/IJSRBS/V6I1.97104.
|
Copyright © by authors and 50Sea. This work is licensed under Creative Commons Attribution 4.0 International License. |




















