Isolation of Keratinolytic from Chicken (Gallus gallus domesticus) Farms and Assessment of their Efficacy in Feathers Degradation
Shazia Bokhari1, Roheela Yasmeen1, Aisha Waheed Qurashi1, Samiya Habib1, Uzma Rafi2
1Lahore Garrison University, Lahore
2Wuhan University China
Corresponding author’s email: shaziabukhari@lgu.edu.pk, roheelayasmeen@lgu.edu.pk
Citation | Bokhari. S, Yasmeen. R, Qureshi. A. W, Habib. S and Rafi. Uzma, “Isolation of Keratinolytic from Chicken (Gallus gallus domesticus) Farms and Assessment of their Efficacy in Feathers Degradation”. International journal of Innovations in Science and Technology, Vol 3, Issue 4, pp: 142-151, 2021.
Received | Nov 15, 2021; Revised | Dec 21, 2021 Accepted | Dec 22, 2021; Published | Dec 23, 2021
ABSTRACT
Keratinolytic microorganisms and their enzymes are associated with poultry feather degradation. In the present study feathers of Gallus gallus domesticus (chicken) and surrounding dry soil was collected from a private poultry sheds located in Jahman village near Lahore. Bacteria were isolated by using enrichment techniques and screened for their proteolytic activity on skim agar. Isolated Bacteria were colonially, morphologically and biochemically characterized and named as SNC1, SNC2, SNC3, SNC4, SCH1, SCH2, SCH3 and SCH4. Results showed closed similarity of bacterial isolates with bacillus species. Effect of various media (LB-broth and Nutrient broth), pHs (7 and 8) and temperatures (4, 37, and 50℃) were recorded on bacterial growth and feather degradation. Bacterial cell densities and amount of keratin produced per gram feather weight were high at temperature 50℃ and pH 8.0. The feather degradation by bacterial isolates was confirmed at different time intervals using stereomicroscopes. The protein analysis of G. gallus domesticus feathers showed protein contents of 3.125g/100 ml. It was concluded high temperature and alkaline pH favored keratin production by bacterial consortia. Moreover, the bacterial isolates used in the current study have the potential to degrade poultry feather waste and extracted keratin is found to be promising for further exploitation of poultry waste.
Keywords: Gallus gallus domesticus, feather biodegradation, keratin waste, Bacillus sp.
- INTRODUCTION
Poultry industry is growing worldwide progressively and generation of waste products from this sector is becoming an environmental issue. These wastes could cause serious soil and water pollution if not processed and discharged properly [1]. Keratinous waste like horns, feather, nails, hoofs, scales, and wools are produced from poultry and meat processing units, slaughterhouses, tanneries and are getting accumulated in the environment [2]. Feathers are considered major waste products that are made up of over 90 % proteins, mainly of beta-keratin which is insoluble and has a very high stability, thus, low degradation rates and can only be degraded by keratinase enzyme [3,4,5]. According to an estimate large quantity of feathers equal to 40 × 109 kg are being produced around the world per year [6]. Nowadays recycling units are installed to make important products such as feather meal This feather meal is multipurpose and used as an animal feed, fertilizer and also to produce environment friendly biodiesel [7]. However, only little amount of feathers are processed and are turned into useful products [8]. Furthermore, feathers are being used in cosmetics, textile, plastic and paper industries biopharmaceutical, biomedical and bioenergy companies [9]. Keratin degrading bacteria and their enzymes also have the potential to improve livestock feed resources [10, 11, 12].
The role of keratin in the feather is to make it resistant against proteases such as trypsin, pepsin and papain. The presence of disulfide bonds back off its degradation process in nature [12, 13, 14]. Keratinase is an extracellular enzyme used for the biodegradation and could only be produced by keratinolytic microorganisms in the presence of keratin substrate [15]. Various keratinolytic microorganisms have been already identified in the literature: some species of Bacillus (B. licheniformis [16], B. subtilis [17] B. cereus [18], B. pumilus [19], actinomycetes and fungi (Aspergillus sp. Penicillin sp. and Cladosporium sp.) [20, 21, 22].
Kumar et al., (2016) have been reported Bacillus species to display keratinolytic activity in soil and poultry compost. Keratinolytic activity is related to both, gram negative (Burkholderia, Chryseobacterium and Pseudomonas) and gram positive (Microbacterium sp.) genera [23, 24]. However, there is still a gap of knowledge regarding the improvement of the efficiency of keratinolytic bacteria which can be enhanced by environmental conditions. The present study aimed to isolate and characterize keratinolytic bacteria associated with poultry farm soil. Furthermore, they were seen further with varying cultural conditions to see their optimum enzyme production in minimum time period.
- MATERIALS AND METHODS
Collection of Feathers and Soil Samples
Feathers of G. gallus domesticus (chicken) along with dry soil (10 gram soil) were collected in sterile plastic bag in winter season from a private poultry sheds in Jahman village located near Lahore. Its coordinates were 31˚ 21 0 N and 74˚ 32 0 E. Samples were collected in the month of December, 2017 and stored at 4˚C.
Preparation of Feathers Powder from chicken (G. gallus domesticus)
Feathers were washed three to four times with sterile water and autoclaved, for 15 minutes at 121℃. Later on they were further dried in hot air oven for 4 hours at 50℃ and crushed in a grinder machine until a powder form was obtained. The yield of keratin powder obtained from feathers was calculated using the following formula:
Yield of Keratin powder (grams) = Amount of feather powder (gram)/ Amount of feathers used * 100
Isolation and Screening of Bacterial Isolates from chicken (G. gallus domesticus) Feather
Bacterial isolation of poultry soil samples was carried out by culture enrichment technique by following the modified method of Kumar et al., (2016). For this purpose, soil (5 gram) was weighed and poured into 250 ml flasks supplemented with 100 ml keratin media (10 g feather powder; 0.5 g NaCl; 0.3 g K2HPO4; 0.4 g KH2PO4; 0.1 g MgSO4.6H2O; pH was adjusted to 7.5 using pH meter). The flasks were incubated for one week at 37℃ at non shaking incubator (SKI 4 Innova Bio-Meditech). Later on, 10 ml culture broth from these enriched flasks (containing feather powder) were transferred into a fresh keratin media with freshly added feathers as a carbon source (2.51 grams) and incubated for 4 weeks at 37℃. The sample from enrichment flasks were serially diluted to 106 and spreaded on nutrient agar medium. Different colonies were obtained and were further purified by quadrant streaking on the same medium in petri plates. Colony morphology of purified cultures of bacteria were recorded.
Qualitative Screening of Bacterial Isolates for Proteolytic Activity using Skim Milk Assay
Purified colonies were further tested for their proteolytic activity by sub culturing isolates on Skim milk Agar (Hi Media MV763). Five grams agar media was taken with 250 ml water in 500 mL flask and allowed to stand for about 15 minutes. Then the media was placed in a cold water bath and was heated gently with frequent shaking to dissolve the medium completely. Media was sterilized by autoclaving at 15 lbs pressure (121℃) for 15 minutes. Then, it was mixed well and poured into sterile Petri plates. Plates were incubated for a week at 37℃. Bacterial colonies with clearing zones on these plates were used for further characterization. Eight isolates were selected on the basis of zone size and named as SNC1, SNC2, SNC3, SNC4, SHC1, SCH2, SCH3 and SCH4.
Effect of Different Media, pH and Temperatures on Bacterial Growth
To study the effect of varying pHs (7 and 8), temperature (4, 37 and 50℃) and media (L-broth and N broth) on bacterial cell densities, bacteria were cultured in L-broth and N broth adjusted at respective pH using pH meter (one Normal HCl or NaOH) and incubated at respective temperature conditions for 24 hours at shaking incubator. Bacterial growth was determined by taking OD at 600 nm using spectrophotometer (China N6000splus).
Images Analysis using Stereo micrograph and Protein Estimation
The result of feathers degradation was visualized after 4 weeks with stereomicroscope. Protein concentration was determined by the method of Lowry et al., (1951). Briefly, protein is estimated by suspending weighed amount of extracted keratin powder in 30 μl of normal saline (0.9%) (Cappucino and Sherman, 2002) followed by the addition of 120 μl phosphate buffer (0.1M) (Cappucino and Sherman, 2002) in 1:4 ratio. The sample was centrifuged at 14000 rpm at 4℃ for 10 minutes and transferred to ice for 2 minutes. Then, 400 μl of supernatant was taken into separate glass tubes and supplemented with 2 ml of folin’s mixture (Cappucino and Sherman, 2002) and allowed to stand at room temperature for 15 minutes. Finally, 0.2 ml of folin’s reagent (commercial grade) was added into it and its OD was measured at 750 nm through spectrophotometer (Lowry et al., 1951). The Bovine Serum Albumin (BSA- commercial grade) was used as a standard. The optical density (OD) was read at 750 nm. Protein content is quantified by comparing OD with standard curve of BSA. A standard curve was made by using conc. of BSA ranging 0.1, 0.08, 0.06, 0.05, 0.04 mg/400 μl of saline solution. These different concentration of BSA were prepared in normal saline and subsequently treated in the same way as for keratin powder.
Colonial, Morphological and Biochemical Characterization of the Bacterial Isolates
Bacterial isolates were characterized colonially and morphologically (gram staining, spore staining and capsule staining) following Cappuccino and Sherman, (2002) techniques. Different tests such as Vogues Proskauer (VP), Methyl red, Catalase, Urease, Indole production, Citrate utilization, DNase test, H2S production and motility tests were performed.
Statistical Analysis
Statistical analysis was carried out between different variables such as temperature and pH using SPSS version 22.0. One way ANOVA was applied on variable temperature treatments. While an independent sample t test was used to analyze pH differences.
- RESULTS AND DISCUSSION
In the present study soil samples were collected from a poultry site, screened and eight bacterial isolates (SNC1, SNC2, SNC3, SNC4, SCH1, SCH2, SCH3 and SCH4) were selected on the basis of clear zones that were produced on skim milk agar (Figure 1).

Figure 1. G. gallus domesticus feather powder
It was noticed in the present study that poultry soil was enriched with microbial diversity with the ability to degrade complex organic compounds. Our findings were in agreement with various studies that showed association of microbes as biodegraders in poultry farms soil [25, 26, 27, 28 29]. Feather degrading bacteria were reported from diverse ecosystems that have a great potential to degrade keratin, however the soil rich in feather is of a great importance due to the excess of keratin [30, 31, 32]. In the study of Lucas et al., (2003), they isolated 33 biodegradable bacteria from dry meadows soil. Bach et al., (2011) also isolated feather-degrading bacteria (Bacillus, Aeromonas and Chryseobacterium genera) from Brazilian soils. In this study G. gallus domesticus feather powder was used for the determination of the keratinolytic activity of keratin degrading bacteria. The feather powder was made of 52.5% protein yield and it was black in color (Figure 2). Protein yield (52.5%) that was identified comparable to protein yield range provided by Lucas et al., (2003) who reported 40-98 %.
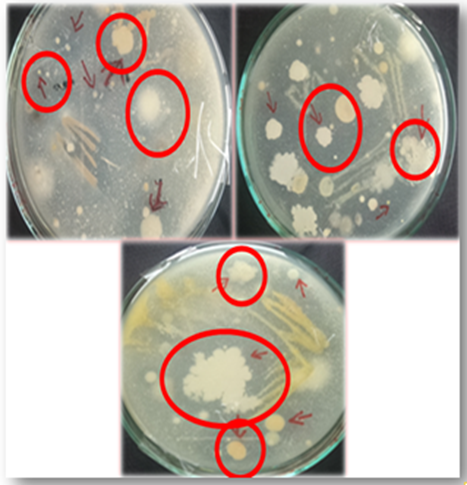
Figure 2. Clear zones on skim milk agar due to proteolytic activity by bacterial isolates (30) obtained from G. gallus domesticus
The effect of varying physiological conditions such as type of media (L-broth and N broth), temperature (4, 37 and 50˚C) and pH (7 and 8) influenced on bacterial growth. Bacterial growth in terms of cell densities were high at temperature 50˚C (106 CFU/ml) as compared to 4˚C and 37˚C (Figure 3).

Figure 3. Effect of different temperatures (4, 37 and 50℃) on bacterial growth
The increase in temperature reduced the degradation time of feathers from 4 weeks to almost 3 weeks (Figure 4).
Figure 4. Effect of temperature on degradation of G. gallus domesticus feathers (with 10 days interval)
Data was statistically analyzed for one way ANOVA and significant difference (p- value < 0.05) in bacterial growth was noticed at 50℃ temperature as compared to 4 and 37℃. Similarly, bacterial growth was high on pH 8.0 with SCH2 and SCH3 bacterial isolates as compared to pH 7.0, although a maximum bacterial growth was recorded by SNC4 at pH 7.0 (Figure 5).
Figure 5. Effect of different pHs (7.0 and 8.0) on bacterial growth
A significant difference in bacterial growth was recorded at pH 8 (p- value < 0.01) using independent sample t test. The increase in pH during cultivation is an important characteristic accompanying keratin hydrolysis and the keratinolytic potential of micro-organisms. Results showed that the increase in temperature and pH causes higher degradation rates (Figure 6).

Figure 6. Effect of pH on degradation of G. gallus domesticus feathers (with 10 days interval)
Abdel-Fattah et al., (2018) also reported that the biodegradation of feather was greater at a temperature range between 50-60℃ and pH between7-9 with keratinase ALW1 isolated from Bacillus licheniformis. Another study with Bacillus species showed best degradation rates between temperatures of 40-50℃ and at 10 pH [33, 34].
Moreover, a comparison was made between samples (flasks with feathers) in the presence and absence of bacterial inoculum and with non-inoculated samples. Bacterial inoculation resulted in a pronounced degradation and the time of degradation was reduced by 10 days as compared to non-inoculated samples. Organisms with a higher keratinolytic activity turned the media into more alkaline, in comparison with other organisms exhibiting lower keratinolytic activity [35, 36, 37]. The protein concentration of G. gallus domesticus feathers was recorded as 3.125g/100 ml and according to the study of Poovendran et al., (2011) the maximum concentration achieved was 7 g/100 ml, which was twice as compared to our results. However, 4.36 g/100 ml protein concentration was reported by Iqtedar et al., (2017) and 4mg/ml was reported by Pandian et al., (2012), whose values were close to our results. The selected isolates were studied for their colonial, morphological and biochemical characteristics and were Gram positive bacteria, spore formers bacteria and rod shaped bacteria. Different tests such as VP (Vogues Proskauer), Catalase, Citrate utilization, and motility turned to be positive. Based on these results of biochemical and morphological characteristics, isolates showed resemblance to Bacillus spp. This was also reported by Mazotto et al., (2011). Bacillus strains are able to produce keratinases in the presence of diverse keratinic waste [38, 39] for example from leather industry, barber shop (Kumawat et al., 2017). The majority of reports studying a variety of keratin-degrading actinobacteria and other bacteria, include Bacillus sp. [40, 41]. Bacillus spp. have been reported to display keratinolytic activity in soil and poultry compost. Bacillus spp. have the ability to degrade 50 g/L of chicken feather and produce amino acids and other products with 70 % of conversion rate in 48 hours.
The pattern of feather degradation was observed at different time intervals using stereomicroscopes and shown in figure 7. Degradation of feather was completed at the fourth week and white powdery mass was observed.
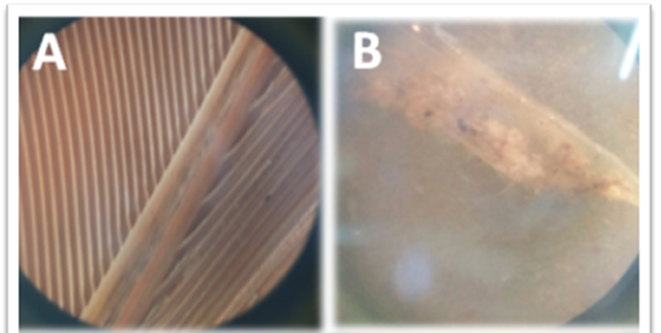
Figure 7. A = G. gallus domesticus feather stereo micrograph; B = stereo micrograph of degradation of G. gallus domesticus feathers after 4 weeks.
- CONCLUSION
In the present study bacterial isolates which was mostly Bacillus spp. were able to degrade G. gallus domesticus feathers from poultry waste using indigenous isolates preferably at high temperature (50℃), and normal pH (8.0 pH). The total protein contents of degraded feather was 3.125g/100 ml. The efficacy of presently used bacterial isolates were found to show the enough potential in degrading poultry waste. Moreover, extracted keratin in present study was promising for further exploitation of industrial use besides poultry waste removal.
ACKNOWLEDGMENTS
We are thankful and acknowledge with deep gratitude to our Head of Department Col (Retd) Dr. Muhammad Amjad Khan for his support during this research work.
CONFLICT OF INTEREST
Authors declare there is no conflict of interest.
REFERENCES
- Abdel-Fattah, A.M., El-Gamal, M.S., Ismail, S.A., Emran, M.A., Hashem, A.M., “Biodegradation of feather waste by keratinase produced from newly isolated Bacillus licheniformisAlw1”Genet EngBiotechn N., 16(2): 311-318.
- Agrahari, S., Wadhwa, N., “Degradation of chicken feather a poultry waste product by keratinolytic bacteria isolated from dumping site at Ghazipur poultry processing plant”Int J Poult Sci., 9(5): 482-489.
- Avdiyuk, K. V., Varbanets, L.D., “Keratinolyic enzymes: producers physical and chemical properties”Application for biotechnology BiotechnologiaActa., 12(2): 27-45.
- Bach, E., Cannavan, F.S., Duarte, F.R., Taffarel, J.A., Tsai, S.M., Brandelli, A. “Characterization of feather-degrading bacteria from Brazilian soils”2011. , 65(1): 102-107.
- Brandelli, A., “Bacterial keratinases: useful enzymes for bioprocessing agroindustrial wastes and beyond” 2008.Food Bioprocess Tech, 1(2): 105-116.
- Cappuccino, J.G., Sherman, N “Techniques for isolation of pure cultures.Cultural Characteristics of Microorganisms, Microbiology A Laboratory Manual, Pearson Education” (2002)-6: 13-23.
- Dalee, A.D., Chehama, M., Sali, K., Hayeeyusoh, N., Hayeewangoh, Z. “Keratinase-producing fungi from local environmental samples of Far South Thailand and their efficiency in hydrolyzing keratinous wastes” In Journal of Physics: Conference Series1097(1): 012036. IOP Publishing.
- Desai, S.S., Hegde, S., Inamdar, P., Sake, N., Aravind, M.SI “solation of keratinase from bacterial isolates of poultry soil for waste degradation”2010.Eng Life Sci., 10(4): 361-367.
- Fakhfakh-Zouari, N., Hmidet, N., Haddar, A., Kanoun, S., Nasari, M.. “A novel serine metallokeratinase from a newly isolated Bacillus pumilus A1 grown on chicken feather meal: biochemical and molecular characterization” ApplBiochem Biotech., 162: 329–344. doi: 10.1007/s12010-009-8774-x.
- Godbole, S., Pattan, J., Gaikwad, S., Jha, T., “Isolation, Identification and Characterization of Keratin degrading microorganisms from Poultry soil and their Feather degradation Potential.”2017.. IJEAB, 2(4).
- Gupta, S., Singh. R.,. “Hydrolyzing proficiency of keratinases in feather degradation”. 2014. Indian J. Microbiol. 54:466–470. doi: 10.1007/s12088-014-0477-5.
- Gurav, R. G., Jadhav, J.P.,. “Biodegradation of keratinous waste by Chryseobacteriumsp. RBT isolated from soil contaminated with poultry waste” 2013. Basic Microbiol., 53(2): 128-135.
- He, Z., Sun, R., Tang, Z., Bu, T., Wu, Q., Li, C., Chen, H.. “Biodegradation of Feather Waste Keratin by the Keratin-Degrading Strain Bacillus subtilis 8” 2018. J MicrobiolBiotechn, 28(2): 314-322.
- Iqtedar, M., Qazi, J. I., Baqri, N., Mirza, S. S., Abdullah, R., Kaleem, A., Naz, S.,. “Bioconversion of agro-industrial feather waste utilizingthermophilicBacillus megatarium” 2017, Rom Biotech Lett, 22(1): 12234-12239.
- Kim, J. M., Lim, W. J., & Suh, H. J. “Feather-degrading Bacillus species from poultry waste”. (2001). Process Biochemistry, 37(3), 287-291.
- Kondamudi, N., Strull, J., Misra, M., Mohapatra, S.K. “A green process for producing biodiesel from feather meal”. Journal of agricultural and food chemistry . 2009. 57(14):6163-6.
- Kumawat, T.K., Sharma, A., Bhadauria, S.,. “Chrysosporiumqueenslandicum: a potent keratinophilic fungus for keratinous waste degradation”. International Journal of Recycling of Organic Waste in Agriculture, (2017) 6(2): 143-148.
- Kumar, M., Kumar, R., & Malik, D. K. “Keratin degradation by bacterial strain isolated from poultry farm soil” (2016).. J. Pharm. Res, 10, 113.
- Laba, W., Rodziewicz, A., “Biodegradation of hard keratins by two Bacillus strains”. 2014.Jundishapur J Microbiol7(2).
- Laba, W., Choinska, A., Rodziewicz, A., Piegza, M., “Keratinolytic abilities of Micrococcus luteus from poultry waste”. Braz J Microbiol,46(3): 691-700.
- Lakshmi, P.J., Chitturi, C.M.K., Lakshmi, V.V., “Efficient degradation of feather by keratinase producing Bacillus sp”. Int. J. Microbiol. doi:10.1155/2013/608321
- Lange, L., Huang, Y., Busk, P.K “Microbial decomposition of keratin in nature—a new hypothesis of industrial relevance”. 2016.Applmicrobiolbiot,100(5): 2083-2096.
- Lateef, A., Adelere, I. A., Gueguim-Kana, E.B., “Bacillus safensis LAU 13: a new source of keratinase and its multi-functional biocatalytic applications”. 2015. BiotechnolBiotechnol Equip, 29(1): 54-63.
- Li, F., Cheng, S., Yu, H., Yang, D., “Waste from livestock and poultry breeding and its potential assessment of biogas energy in rural China”. 2016. Clean. Prod., 126: 451-460.
- Lowry, O.H., Rosebrough, N.J., Farr, A.L., Randall, R.J., 1951. “Protein measurement with the Folin phenol reagent”. 1951.Jbiolchem,193(1): 265-275.
- Lucas, F. S., Broennimann, O., Febbraro, I., Heeb, P., “High diversity among feather-degrading bacteria from a dry meadow soil”. 2003. Ecol., 45(3): 282-290.
- Mazotto, A.M., de Melo, A.C.N., Macrae, A., Rosado, A.S., Peixoto, R., Cedrola, S.M.,Couri, S., Zingali, R.B., Villa, A.L., Rabinovitch, L.,Chaves, J.Q. “Biodegradation of feather waste by extracellular keratinases and gelatinases from Bacillus spp”. 2011. World J MicrobBiot,27(6): 1355-1365.
- Onifade, A. A., Al-Sane, N. A., Al-Musallam, A. A., Al-Zarban, S., “A review: potentials for biotechnological applications of keratin-degrading microorganisms and their enzymes for nutritional improvement of feathers and other keratins as livestock feed resources”. Bioresour. Technol, 66(1): 1-11.
- Pandian, S., Sundaram, J., Panchatcharam, P., “Isolation, identification and characterization of feather degrading bacteria”. 2012. J. Exp. Biol., 2(1): 274-282.
- Peng, Z., Mao, X., Zhang, J., Du, G., Chen, J., “Effective biodegradation of chicken feather waste by co-cultivation of keratinase producing strains”. 2019. M Cell Fact. 18(1): 84.
- Poovendran, P., Kalaigandhi, V., Kanan, V. K., Rani, E. J., Poongunran, E., “A study of feather keratin degradation by Bacillus licheniformis and quantification of keratinase enzyme produced”. 2011. J MicrobiolBiotechnol Res, 1: 120-126.
- Riffel, A., Brandelli, A., “Keratinolytic bacteria isolated from feather waste”. Braz J. Microbiol., 37(3): 395-399.
- Riffel, A., Lucas, F., Heeb, P., Brandelli, A., “Characterization of a new keratinolytic bacterium that completely degrades native feather keratin”. Arch. Microbiol, 179(4): 258-265.
- Sekar, V., Kannan, M., Ganesan, R., Dheeba, B., Sivakumar, N., Kannan, K., “Isolation and screening of keratinolytic bacteria from feather dumping soil in and around Cuddalore and Villupuram, Tamil Nadu”. 2016.Proceedings of the National Academy of Sciences, India Section B: Biological Sciences, 86(3): 567-575.
- Singh, S.,Masih, H., “Isolation of keratinolytic bacteria from soil for the bioconversion of the poultry feather waste”. 2015. JPure ApplMicrobio, 9(3), 2281-2284.
- Swetlana, N., Jain, P.C., “Feather degradation by strains of Bacillus isolated from decomposing feathers”. 2010. Braz J Microbiol, 41(1): 196-200.
- Tesfaye, T., Sithole, B., Ramjugernath, D., “Valorisation of chicken feathers: a review on recycling and recovery route—-current status and future prospects”. 2017 c. Clean TechnolEnvir, 19(10): 2363-2378.
- Tesfaye, T., Sithole, B., Ramjugernath, D., Chunilall, V.,. “Valorisation of chicken feathers: characterisation of chemical properties”. 2017 a. J. Waste Manag. 68: 626-635.
- Tesfaye, T., Sithole, B., Ramjugernath, D., &Chunilall, V., “Valorisation of chicken feathers: application in paper production”. 2017 b. Clean. Prod, 164: 1324-1331.
- Veenayohini, K., Sangeetha, D., “Isolation and identification of keratinolytic bacteria from poultry waste and assessment of its keratinase activity on chicken feathers”. 2016. J. Appl. Eng. Res., 2(11): 396-402.
- Xu, B., Zhong, Q., Tang, X., Yang, Y., Huang, Z., “Isolation and characterization of a new keratinolytic bacterium that exhibits significant feather-degrading capability”. 2009. Afr. J. Biotechnol., 8(18):4590–4596.




















