Role of Microorganisms in Modern Food Industry
Sumaira Mazhar1, Roheela Yasmeen1, Afeefa Chaudhry1, Khadija Summia1, Ibrar Hussain1, Sadia Amjad1, Ehtisham Ali1
1Department of Biology, Lahore Garrison University, Lahore
*Corresponding author’s Email ID: roheelayasmeen@lgu.edu.pk
Citation | Mazhar. S, Yasmeen. R, “Role of Microorganisms in Modern Food Industry”, International Journal of Innovations in Science and Technology. Vol 4, Issue 1, pp: 65-77, 2022
Received | Nov 15, 2021; Revised | Dec 21, 2021 Accepted | Dec 23, 2021; Published | Jan 28, 2022.
Abstract
Microorganisms are an important part of the food industry as these are helpful in food preservation and production. Usually, microorganisms are used in making dairy products (yogurt and cheese), fermented vegetables (olives, pickles, and sauerkraut), fermented meats (salami), and sourdough bread. These are also utilized for the production of wine and several other beverages. Recently in the food industry, the use of microorganisms has started on a large scale for the production of chocolate, food color, from preserving fruits, vegetables and meat, and as probiotics which are helpful for human health. Different types of the microorganisms produce enzymes of nutritional value such as microbial transglutaminase for fish production. As the human population is increasing, we need to adopt new techniques for producing qualitative and nutritious food. These microorganisms can be used to cope with the shortage of food supply. This review will brief the role of microorganisms in above mentioned products as a leading step towards the modern food industry.
Keywords: Fermentation, Lactic acid, Nisin, Probiotics, Transglutaminase
INTRODUCTION
Most of the microorganisms including bacteria and fungi have been used to obtain food, antibiotics and other products since ages. Moreover, microorganisms contribute to the smell, texture and taste of the food and cause spoilage of the food. One such type of bacteria are Lactobacilli that is used for the production of food as these bacteria ferment lactic acid [1]. Lactobacilli are conventionally used to add aroma and texture to the food while preventing the spoilage of dairy products, meat and vegetables as well as silage [2]. Fermented foods not only have high nutritional value but their shelf life is also extended. Moreover, due to increased acidity, these food products do not harbor pathogenic microorganisms [3,4]. Fermented foods not only have antitumor [5] and anticholesterol activities [6,7] but also reduce nitrites and reduce gastrointestinal (GI) disorders [8, 9].
Microorganisms that are present in the GI tract are known for producing health promoting compounds that are called probiotics. These probiotics in fermented products help in the preservation of milk products through the formation of lactic acid which adds to the flavor as well as nutritional value of the food [10]. It is also reported in many epidemiological studies that high intake of fruits and vegetables helps to reduce the incidence of chronic diseases [11-13]. These foods provide phytochemicals, fibers and antioxidants that reduce the risk of cell damage and contribute to the better health of the consumer [14].
One emerging field related to food and food products is the extraction of food colors from microbial sources. There are many mechanisms that are responsible for a significant color to a specific species of microorganisms. Prior to the extraction of microbial colors, the safety and efficacy are measured and it is confirmed that these colors are edible and non-toxic [15]. Fungal cells offer a reliable source for extraction of color and their use in foods [16]. Other than these properties, microorganisms produce chemical agents such as Nisin that have antimicrobial properties; however, these compounds can be decomposed by digestive enzymes. Nisin is being used to prevent spoilage of cheese from Clostridium tyrobutyricum [17]. Nowadays it is also being used to preserve and produce various types of cheese, ready-made soups and canned foods [18, 19]. The role of different types of microorganisms in various food processes (Table 1) has been reviewed in this paper.
Table 1: Microbial activity of different microbes used in various industries.
| Sr. No. | Microbial activity | Microbes | Use in industry | References |
| 1. | Pectinolytic activity | Lactobacillus brevis L166, Erwinia herbicola C26, B. subtilis C12, and Kluyveromyces fragilis K211 | Cofee industry | [72] |
| 2. | Naringinase activity | Aspergillus niger, Aspergillus oryzae, Penicilium decumbens, Phanopsis citri, Aspergillususamii, Cochiobolus miyabeanus, Rhizotonia solani and Rhizopus nigricans | Fruit juices industry | [73] |
| 3. | Fermentation | Bothrytiscinerea spp.Streptococcus thermophilus and Lactobacillus bulgaricus | Dairy industry | [74] |
| 4. | Protease activity | Bacillus tequilensis strain SCSGAB0139 | Brewing industry | [75] |
| 5. | Asparaginase activity | Cladosporium sp | Baking industry | [73] |
Methodology
For this review, different articles from Google Scholar, Wiley library and PubMed library were searched. More than 95 articles including books, case reports and case series were searched. However, out of 95, 75 article that were found related to our topic considered for the present review article.
Role of Lactic Acid Bacteria
Lactic acid bacteria are being used in a number of food production and storage methods in the modern food industries. Lactobacilli are commonly used for the storage of uncooked fermented sausages, hams and sliced meats to avoid pathogenic Listeria infection [20,21]. These bacteria have replaced the chemical additives such as sodium lactate and potassium acetate which were used for the safety and quality of the above mentioned meat products; however, these additives leave numb mouth feel or warmed-over flavor upon eating [22].
The basic mechanism of action of lactic acid bacteria in raw fermented sausages is the conversion of sugars to lactic acid through fermentation. This contributes to the unfavorable conditions for the growth of pathogenic and spoilage microorganisms. Other than the production of lactic acid, these bacteria produce small amounts by products such as acetic acid, acetoin, ethanol and pyruvic acid which act as the precursors for production of other compounds besides adding aroma to the food [23,24].
Lactic acid producing bacteria such as Wiessella spp. and Limosilactobacillus spp have the ability of producing certain exopolysaccharides that are recognized as safe food industry by the Food and Drug Administration [25]. One such homopolysaccharide called α-ᴅ-glucans is produced by Leuconostoc spp. and Lactobacillus spp. that is frequently used in baking industry to improve softness and texture of baked products such as breads [26]. It is also used as a stabilizer and emulsifier in ice cream product while preventing the crystallization during freezing. It has also been reported that common lactic acid producing bacteria such as Lactobacillus and Streptococcus spp. produce β-ᴅ-fructans is used as a prebiotic and also a replacement for sugars [27]. Exopolysaccharide produced by Lactic acid producing bacteria is also used to improve texture and shelf life of plant based dairy like products in vegan industry [28].
Role of Microbial Transglutaminase
Transglutaminase is an enzyme that is involved in the catalysis of acyl- transfer reactions between proteins, peptides and amines [29]. Generally, transglutaminase is found in plant and animal tissues [30-32]. A study conducted to screen 5000 bacteria from soil samples showed that many soil bacteria have capability of producing transglutaminase. These bacteria include Streptoverticillium strains such as S. griseocarneum, S. cinnamoneum and S. mobaraense [33,34].
Transglutaminases are commonly used in fish products such as Japanese fish paste. Moreover, the fish paste produced by treatment of transglutaminase had better texture [35-37]. Transglutaminase also has the ability to maintain the freshness of vegetables and fruits when a coat of transglutaminase is applied to them. One such study showed that when cut celery was coated with transglutaminase, it not only preserved the freshness of celery but also reduced the bacterial growth [38].
Besides the preservative properties of transglutaminase, it is also evident that it reduces the allergenicity of some proteins and peptides such as casein [39]. Other than the preservative applications of microbial transglutaminase, it is also used to improve the quality of dough’s made by poor qualify flour and results in improved baking and texture of the bread. It also improves the pore size and elasticity of the baked bread which in turn increases the shelf life of the bread.
Microorganisms and Chocolate Production
Chocolate probably originated in Mesoamerica and is being used as food, medicine, and beverage since the past 2,000 years. Researches showed that raw cocoa is rich in antioxidants which are also present in vegetables and tea. These antioxidants not only provide defense against cancer causing reactive oxygen species but also prevent oxidation of LDL-cholesterol [40,41]. The role of microorganisms is to enhance the flavor of chocolate through fermentation when the pod of the cocoa bean is opened which leads to a less astringent and bitter taste of the chocolate. This method is greatly used for qualitative enhancement of some low quality Indonesian cocoa beans [42]. Such microbial fermentation is usually performed at level of farms with a quantity of cocoa beans ranging from about 25 to 2000 kg that are enclosed in banana leaves. The type of microorganisms that assist in this fermentation depends upon the local environment and climatic conditions [43]. There are two types of fermentation taking place during the production of chocolate from raw cacao beans. First, the alcoholic fermentation that is mediated by the yeast which kills the bean and enhances the flavor of chocolate by converting sugar into alcohol [44]. The second fermentation is acetic acid fermentation in which alcohol is oxidized and acetic acid is produced which overall improves the texture, aroma, flavor and color of the chocolate [45].
Microorganisms used in the production of Biocolors
Colors that are used in foodstuff, dyes, cosmetics and other industries are usually synthetic and possess toxic properties. These chemical colors also have carcinogenic properties, so production of colors and pigments from biological resources is very interesting topic of research among scientists these days [46]. Various types of microorganisms including fungi, algae and yeasts are colored in nature and their color can be extracted for industrial purposes. Currently, fungi are considered as an important source of biocolors [47]. Therefore fungal carotenoids, and polyketide azaphilone have been approved by the European Union for pigment production [48, 49]. The use of fungal colorants allows the manufacturers to use these colorants when seasonal supply of chemical colorants is not available. Species belonging to Monascus genus of fungi produce three different pigments including orange, red and yellow. These pigments are commonly used in East Asian foods such as red rice wine and red bean curd [48, 50].
Other than fungi, Bacillus sp. produces riboflavin that are rich in yellow orange pigment and currently being used in baby foods, cheese, pasta sauce and cereals. However, despite the ability of microbes to produce color pigments, these synthetic pigments cannot replace the natural colors completely. The challenges include high cost of production as all microbes have different growth requirements, low stability of the pigment produced and variation in the shades of color due to slight changes in growth conditions. But the production of colors by fermentation is promising because of low cost, high stability, ease of extraction and higher yields [51].
To minimize the hurdles present between the mass production of bio colors; a combination of genetic modifications and fermentation followed by the ultrasonication of cells in order to extract the color can be used completely [52].
Microorganisms as Probiotic
The word probiotic means “for life” and in microbiology, it can be defined as the alive microorganisms that provide health benefits to humans which cannot be attained by general nutrition. Probiotics are currently of great interest in industries such as food, medicine and dietary supplements [53].
Dairy drinks are the first rich source of probiotics and are also called fortified dairy products. Bifidobacterium and Lactobacillus are the bacteria that grow poorly in milk because of their inability to utilize lactose as a carbon source. To ease this difficulty, enhancers are added to the milk such as citrus fiber to increase the growth of probiotics present in the fermented milks [54].
Yogurt is considered the natural source of probiotics as it is produced by Lactobacillus and Streptococcus species. These probiotics are often thought to exhibit as sources of certain effects on yogurt such as acidity, flavor, texture and appearance. Therefore, it is safe to say that addition of probiotics and cysteine can improve the stability and other characteristics of yogurt [55].
Other than dairy products, there is also a demand for the use of probiotics in vegetables and fruit juices. It is because of the inability of a large population to digest the different components present in dairy. In plant based substrates, probiotics need 37℃ temperature to grow which makes the production of non-dairy probiotic products [56]. In case of fruit juices, several probiotic strains are used depending on the substrate that allow the fermentation of fruit juices without altering the flavor. While, in case of fermented pomegranate juice, Lactobacillus plantarum and Lactobacillus delbruekii prove to be the desirable probiotics [57].
Role of microorganisms in the processing and preservation of Meat
In meat industry, the main challenge is the preservation of raw meat and meat products for a longer period of time. To achieve this, antagonistic cultures of bacteria are used to restrict the growth of pathogenic and the spoilage microbes. These cultures are usually termed as protective cultures [58].
As described earlier, Lactic acid bacteria are primarily used in preservation of meat products. The main mechanism of action of these bacteria is to produce lactic acid and acetic acid through fermentation that lowers the pH of the meat product. This leads to the preservative effect that is desired to be achieved [59].
Besides the production of acids, these bacteria produce bacteriocins that have antimicrobial properties. These are the peptides that can inhibit the growth of Listeria on meats and also have limited activity against spore formers such as Bacillus and Clostridium. Since bacteriocins are peptides in nature that are cleaved by proteases present in the intestines; therefore, there is no safety concern for their use in meat industry [60]. Despite all the advantages, there are some drawbacks linked with the use of bacteriocins. The bacteriocins are least effective in solid medium or solid foods which makes its use limited to liquid food only. Moreover, if it binds with certain food components, which leads to reduce its activity. The effectiveness of bacteriocin is also dependent on its distribution in food and presence of proteases [61].
Role of microorganisms in Nisin Activity
Nisin is a bacteriocin produced by Lactococcus lactis and is a member of type-I lantibiotic group as it contains an amino acid named lanthionine [18]. Nisin has the ability to prevent the growth and spore formation of many of the gram positive and gram negative bacteria. Some of these bacteria include Listeria monocytogenes, Staphylococcus aureus, Bacillus and Clostridium species, Escherichia coli and Salmonella [62]. Nisin has been extensively studied for its safety as a food preservative and is considered the only bacteriocin that is permitted for use in food [63]. Studies have reported that Nisin is usually more effective against Gram positive bacteria and cannot penetrate lipopolysaccharides present in the cell wall of Gram negative bacteria but its use with a chelating agent such as Ethylenediaminetetraacetic acid (EDTA) increases its effectiveness against Gram negative bacteria [64].
Nisin is commonly used to preserve foods that are low acid canned foods as neutral or near to neutral pH facilitates the growth of spoilage and pathogenic bacteria. These foods generally include canned, minimum heat processed vegetables that have pH above 4.5 [65]. The food products such as crumpets are rich in moisture and favor the growth of bacteria that cause food poisoning. One study showed that untreated crumpets provided suitable conditions for the growth of Bacillus cereus which caused food poisoning in consumers. When Nisin was added to the crumpet batter at the level of 3.75 µg/g, the bacteria was not capable of growth to levels that could cause food poisoning [66]. Other than the use of Nisin in canned vegetables and crumpets, it is also used in cured meat that not only acts as preservative but also helps to reduce the levels of nitrite in cured meat [67].
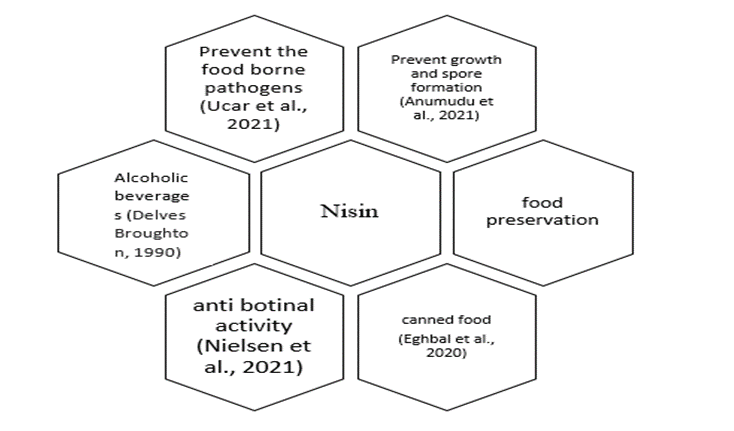
Figure 1. Role of Nisin in food industry
Role of microorganisms in increasing Antioxidant activity
Antioxidants are a group of chemical compounds that inhibit or delay the process of oxidation or and ultimately help in prevention of cell damage [68]. Lately, use of antioxidants have gained importance in nutrition and food industry because it not only increase the shelf life of food while maintaining nutritional quality but also reduces the oxidative damage to the consumer’s body [69, 70, 71].
Commonly used antioxidants are carotenoids such as Beta carotene, lycopene and astaxanthin. Beta- carotene is produced by the mold Blakeslea trispora and algae Dunaliella salina and is used in human foods. Astaxanthin produced by the yeast Xanthophyllomyces dendrorhous is currently being used in fish foods [72]. B. trispora also produces lycopene that is also produced by Streptomyces sp. and is used in human foods as both antioxidant as well as food color [73, 74, 75].
Concluding Remarks
An attempt was made to move that microorganisms are very important and widely used for the welfare of mankind by increasing food production. They are very useful for the modern food industry as they perform various functions to improve the quality of food. Most of the bacteria are used for the preservation of food because of Nisin activity. These microorganisms also produce enzymes like transglutaminase which is very important for preservation of fish products. The lactic acid bacteria produce lactic acid which is used for preservation of a variety of meat and meat products. The role of bacteria in chocolate production is widely studied as it has a great impact in the modern food industry. Bio-coloration is another emerging field of microbial role in the food industry. Microorganisms as probiotics have increased the immunity among humans and this is a great discovery to improve human health. Antioxidant activity of vegetables and fruits is enhanced by using microbes; this is very important for human health.
It is concluded that there is need to focus useful microbes for the welfare of mankind to increase the food production. A number of microbial functions such as preservation, quality enhancement of food products, antioxidant properties and usage as a probiotics makes them best both for human and environment.
Acknowledgement
We are extremely thankful to all authors for their cooperation for timely completion of the work.
Author’s Contribution
Sumaira Mazhar: Main idea, supervisor
Roheela Yasmeen: supervisor, final proof reading and corresponding author
Afeefa Chaudhry, Sadia Amjad: rephrasing and help in data collection
Khadija Summia, Ibrar Hussain, Ehtisham Ali: Collection of data
Conflict of interest
Authors declare there is no conflict of interest for publishing this manuscript in IJIST.
Project details
The aim of this research was to highlight the importance of microbes with reference to food industry.
REFERENCES
- Naghmouchi, K., Belguesmia, Y., Bendali, F., Spano, G., Seal, B. S., & Drider, D., “Lactobacillus fermentum: a bacterial species with potential for food preservation and biomedical applications” Critical reviews in food science and nutrition, 60(20): 3387-3399.
- Ayivi, R. D., Gyawali, R., Krastanov, A., Aljaloud, S. O., Worku, M., Tahergorabi, R., Silva R. C., Ibrahim, S. A. “Lactic Acid Bacteria: Food Safety and Human Health Applications” 2020. Dairy, 1(3): 202-232.
- Steinkraus, K. H., “Fermented foods and beverages: the role of mixed cultures” Bull AT, Slater JH (eds) Microbial interactions and communities, 1: 407-442.
- Hossain, M. I., Kim, K., Mizan, M. F. R., Toushik, S. H., Ashrafudoulla, M., Roy, P. K., ... & Ha, S. D., “Comprehensive molecular, probiotic, and quorum-sensing characterization of anti-listerial lactic acid bacteria, and application as bioprotective in a food (milk) model” 2021. Journal of Dairy Science, 104(6): 6516-6534.
- Murooka, Y., & Yamshita, M., “Traditional healthful fermented products of Japan Journal of Industrial Microbiology and Biotechnology, 35(8): 791.
- Ajdari, Z., Abd Ghani, M., Khan Ayob, M., Bayat, S., Mokhtar, M., Abbasiliasi, S., Khoramnia, A., Rahman, H.S., Mehrbod, P., Ajdari, D. & Ariff, A. B., “Hypocholesterolemic activity of monascus fermented product in the absence of monacolins with partial purification for functional food applications” The Scientific World Journal.
- Swain, M. R., Anandharaj, M., Ray, R. C., & Rani, R. P., “Fermented fruits and vegetables of Asia: a potential source of probiotics” Biotechnology Research International.
- Sissons, J. W., “Potential of probiotic organisms to prevent diarrhoea and promote digestion in farm animals–a review” Journal of the Science of Food and Agriculture, 49(1): 1-13.
- Dimidi, E., Cox, S. R., Rossi, M., & Whelan, K., “Fermented foods: definitions and characteristics, impact on the gut microbiota and effects on gastrointestinal health and disease” Nutrients, 11(8): 1806.
- Kechagia, M., Basoulis, D., Konstantopoulou, S., Dimitriadi, D., Gyftopoulou, K., Skarmoutsou, N., & Fakiri, E. M., “Health benefits of probiotics: a review” International Scholarly Research Notices.
- Boeing, H., Bechthold, A., Bub, A., Ellinger, S., Haller, D., Kroke, A., Leschik-Bonnet, E., Müller, M.J., Oberritter, H., Schulze, M. & Watzl, B., “Critical review: vegetables and fruit in the prevention of chronic diseases” European Journal of Nutrition, 51(6): 637-663.
- Corsello, A., Pugliese, D., Gasbarrini, A., & Armuzzi, A., “Diet and nutrients in gastrointestinal chronic diseases” Nutrients, 12(9): 2693.
- del Río-Celestino, M., & Font, R., “The health benefits of fruits and vegetables”
- Slavin, J. L., & Lloyd, B., “Health benefits of fruits and vegetables” 2012. Advances in nutrition, 3(4): 506-516.
- Sen, T., Barrow, C. J., & Deshmukh, S. K., “Microbial pigments in the food industry—challenges and the way forward” Frontiers in nutrition, 6, 7.
- Gupta, C., Garg, A. P., Prakash, D., Goyal, S., & Gupta, S., “Microbes as potential source of biocolours” Pharmacologyonline, 2: 1309-1318.
- Hassan, H., St-Gelais, D., Gomaa, A., & Fliss, I., “Impact of Nisin and Nisin-Producing Lactococcus lactis ssp. lactis on Clostridium tyrobutyricum and Bacterial Ecosystem of Cheese Matrices” Foods, 10(4), 898.
- Gharsallaoui, A., Oulahal, N., Joly, C., & Degraeve, P., “Nisin as a food preservative: part 1: physicochemical properties, antimicrobial activity, and main uses” Critical reviews in food science and nutrition, 56(8): 1262-1274.
- Malvido, M. C., González, E. A., & Guerra, N. P., “Nisin production in realkalized fed-batch cultures in whey with feeding with lactose-or glucose-containing substrates” Applied microbiology and biotechnology, 100(18): 7899-7908.
- Katla, T., Møretrø, T., Sveen, I., Aasen, I. M., Axelsson, L., Rørvik, L. M., & Naterstad, K., “Inhibition of Listeria monocytogenes in chicken cold cuts by addition of sakacin P and sakacin P‐producing Lactobacillus sakei” Journal of Applied Microbiology, 93(2): 191-196.
- McIntyre, L., Hudson, J. A., Billington, C., & Withers, H., “Biocontrol of foodborne bacteria” In Novel technologies in food science (pp. 183-204). Springer, New York, NY.
- Vermeiren, L., Devlieghere, F., Vandekinderen, I., Rajtak, U., & Debevere, J., “The sensory acceptability of cooked meat products treated with a protective culture depends on glucose content and buffering capacity: A case study with Lactobacillus sakei 10A” Meat science, 74(3): 532-545.
- Fontana, C., Fadda, S., Cocconcelli, P. S., & Vignolo, G., “Lactic acid bacteria in meat fermentations” Lactic Acid Bacteria–Microbiological and Functional Aspects, 4th Ed. CRC Press, Taylor & Francis, Boca Raton, London, New York, 247-264.
- Hui, Y. H. (Ed.). “Handbook of meat and meat processing” CRC press.
- Angelin, J., & Kavitha, M “Exopolysaccharides from probiotic bacteria and their health potential”, “International Journal of Biological Macromolecules” 2020, 162: 853-865.
- Xu, D., Hu, Y., Wu, F., Jin, Y., Xu, X., & Gänzle, M. G “Comparison of the functionality of exopolysaccharides produced by sourdough lactic acid bacteria in bread and steamed bread”, Journal of Agricultural and Food Chemistry 2020, 68(33): 8907-8914.
- Korcz, E., & Varga, L., “Exopolysaccharides from lactic acid bacteria: Techno-functional application in the food industry” 2021. Trends in Food Science & Technology.
- Ripari, V., “Techno-functional role of exopolysaccharides in cereal-based, yogurt-like beverages” “Beverages” 2019, 5(1): 16.
- Strop, P., Versatility of microbial transglutaminase Bioconjugate Chemistry, 25(5): 855-862.
- Yasueda, H., Kumazawa, Y., & Motoki, M., “Purification and characterization of a tissue-type transglutaminase from red sea bream (Pagrus major)” Bioscience, Biotechnology, and Biochemistry, 58(11): 2041-2045.
- Akbari, M., Razavi, S. H., & Kieliszek, M., “Recent advances in microbial transglutaminase biosynthesis and its application in the food industry” Trends in Food Science & Technology.
- Alhasani, H. A. W., & Al-Younis, Z. K., “Extraction, Purification and Characterization of Transglutaminase from Some Plants” In IOP Conference Series: Earth and Environmental Science. 910(1): 012061. IOP Publishing.
- Motoki, M., Okiyama, A., Nonaka, M., Tanaka, H., Uchio, R., Matsura, A., Ando H, & Umeda, K., “Novel transglutaminase manufacture for preparation of protein gelling compounds” Jpn Kokai Kokkyo Koho JP, 127471.
- Mostafa, H. S., “Microbial transglutaminase: An overview of recent applications in food and packaging” Biocatalysis and Biotransformation, 38(3): 161-177.
- Ichihara, Y., Wakameda, A., & Motoki, M., “Fish meat paste products containing transglutaminase and their manufacture” Jpn Kokai Tokkyo Koho JP, 2186961.
- Miwa, N., “Innovation in the food industry using microbial transglutaminase: Keys to success and future prospects” Analytical Biochemistry, 597: 113638.
- Sarojini, A., Sukumar, D., & Kumar, V., “Effect of transglutaminase and egg white on functional properties and microstructure of fish paneer from tilapia and common carp” 2020. Journal of Animal Research, 10(6): 931-940.
- Takagaki, Y., Narakawa, K., & Uchio, R., “Coating of vegetables and fruits with transglutaminase and proteins for preservation” Jpn Kokai Tokkyo Koho JP, 3272639.
- Kieliszek, M., & Misiewicz, A., “Microbial transglutaminase and its application in the food industry. A review” Folia microbiologica, 59(3): 241-250.
- Scalbert, A., & Williamson, G., “Chocolate: modern science investigates an ancient medicine” J Med Food, 3(2): 121-125.
- Weisburger, J. H., “Chemopreventive effects of cocoa polyphenols on chronic diseases” Experimental Biology and Medicine, 226(10): 891-897.
- Misnawa, S. J., Jamilah, B., & Nazamid, P., “Polyphenol oxidase refeneration of under-fermented dried cocoa beans and its possibility to improve cocoa flavor quality” In Proc. Of the 13th International Cocoa Research Conference (pp. 1025-1032).
- Schwan, R. F., “Microbial fermentation of cocoa beans with emphasis on enzymatic degradation of the pulp” 1995. In J. Appl. Bacteriol. Symp. Suppl. 79: S96-S107.
- Zhao, J., & Fleet, G “Yeasts are essential for cocoa bean fermentation” “International Journal of Food Microbiology” 2014, 174: 72-87.
- Kalsoom, M., Rehman, F. U., Shafique, T. A. L. H. A., Junaid, S. A. N. W. A. L., Khalid, N., Adnan, M., ... & Ali, H “Biological importance of microbes in agriculture, food and pharmaceutical industry: A review” “Innovare J. Life Sci” 2020, 8(6): 1-4.
- Unagul, P., Wongsa, P., Kittakoop, P., Intamas, S., Srikitikulchai, P., & Tanticharoen, M.. “Production of red pigments by the insect pathogenic fungus Cordyceps unilateralis BCC 1869” Journal of Industrial Microbiology and Biotechnology, 32(4): 135-140.
- Raisanen, R., Nousiainen, P., & Hynninen, P. H., “Dermorubin and 5-chlorodermorubin natural anthraquinone carboxylic acids as dyes for wool” 2002, Textile research journal, 72(11): 973-976.
- Mapari, S. A., Thrane, U., & Meyer, A. S., “Fungal polyketide azaphilone pigments as future natural food colorants?” Trends in biotechnology, 28(6): 300-307.
- Caro, Y., Venkatachalam, M., Lebeau, J., Fouillaud, M., & Dufossé, L., “Pigments and colorants from filamentous fungi” 2017, Fungal metabolites, 499-568.
- Nakanishi, K., Goto, T., & Itô, S. (Eds.). “Natural Products Chemistry”, 2013: Volume 1 (Vol. 1). Academic press.
- Joshi, V. K., Attri, D., Bala, A., & Bhushan, S., “Microbial pigments”
- Lu, Y., Wang, L., Xue, Y., Zhang, C., Xing, X. H., Lou, K., ... & Su, Z., “Production of violet pigment by a newly isolated psychrotrophic bacterium from a glacier in Xinjiang, China” 2009, Biochemical Engineering Journal, 43(2): 135-141.
- FAO/WHO, Expert Consultation., “Health and nutritional properties of probiotics in food including powder milk with live lactic acid bacteria” 2001, World Health Organization Córdoba.
- Saarela, M., Virkajarvi, I., Alakomi, H. L., Sigvart-Mattila, P., & Matto, J., “Stability and functionality of freeze-dried probiotic Bifidobacterium cells during storage in juice and milk” 2006, International Dairy Journal, 16(12): 1477-1482.
- Dave, R. I., Shah, N. P., “Viability of yoghurt and probiotic bacteria in yoghurts made from commercial starter cultures” 1997, International Dairy Journal, 7(1): 31-41.
- Savard, T., Gardner, N., & Champagne, C. P., “Growth of Lactobacillus and Bifidobacterium cultures in a vegetable juice medium, and their stability during storage in a fermented vegetable juice” 2003, Sciences des Aliments (France).
- Sanders, M. E., & Huis, J., “Bringing a probiotic-containing functional food to the market: microbiological, product, regulatory and labeling issues” Lactic Acid Bacteria: Genetics, Metabolism and Applications, 293-315.
- Vermeiren, L., Devlieghere, F., & Debevere, J., “Evaluation of meat born lactic acid bacteria as protective cultures for the biopreservation of cooked meat products” 2004, International journal of food microbiology, 96(2): 149-164.
- Niku‐Paavola, M. L., Laitila, A., Mattila‐Sandholm, T., & Haikara, A., “New types of antimicrobial compounds produced by Lactobacillus plantarum” 1999, Journal of applied microbiology, 86(1), 29-35.
- Jeevaratnam, K, Jamuna, M, & Bawa, AS., “Biological preservation of foods–Bacteriocins of lactic acid bacteria”2005.
- Schillinger, U., Geisen, R., & Holzapfel, W. H., “Potential of antagonistic microorganisms and bacteriocins for the biological preservation of foods” 1996. Trends in Food Science & Technology, 7(5): 158-164.
- Belfiore, C., Castellano, P., Vignolo, G., “Reduction of Escherichia coli population following treatment with bacteriocins from lactic acid bacteria and chelators” 2007, Food microbiology, 24(3): 223-229.
- Federal, R., “Nisin preparation affirmation of GRAS status as a direct human ingredient” 1988, Food Registration, 54: 11247-11251.
- Khan, A., Vu, K. D., Riedl, B., & Lacroix, M., “Optimization of the antimicrobial activity of Nisin, Na-EDTA and pH against gram-negative and gram-positive bacteria” LWT-Food Science and Technology, 61(1): 124-129.
- Delves-Broughton, J. “Nisin and its application as a food preservative” Journal of the Society of Dairy Technology, 43(3): 73-76.
- Jenson, I., Baird, L., & Delves-Broughton, J., “The use of Nisin as a preservative in crumpets” Journal of food protection, 57(10): 874-877.
- Marcos, B., Aymerich, T., Garriga, M., & Arnau, J., “Active packaging containing Nisin and high pressure processing as post-processing listericidal treatments for convenience fermented sausages” Food Control, 30(1): 325-330.
- Yadav, A., Kumari, R., Yadav, A., Mishra, J. P., Srivatva, S., & Prabha, S., “Antioxidants and its functions in human body-A Review” 2016, Research in environment and life sciences, 9(11): 1328-1331.
- Finley, J. W., Kong, A. N., Hintze, K. J., Jeffery, E. H., Ji, L. L., & Lei, X. G., “Antioxidants in foods: state of the science important to the food industry” Journal of agricultural and food chemistry, 59(13): 6837-6846.
- Lee, J. S., Park, S. A., Chung, D., & Lee, H. G., “Encapsulation of astaxanthin-rich Xanthophyllomyces dendrorhous for antioxidant delivery” International journal of biological macromolecules, 49(3): 268-273.
- Noviendri, D., Hasrini, R. F., & Octavianti, F., “Carotenoids: Sources, medicinal properties and their application in food and nutraceutical industry” Journal of Medicinal Plants Research, 5(33): 7119-7131.
- Haile, M., & Kang, W. H., “The role of microbes in coffee fermentation and their impact on coffee quality” Journal of Food Quality.
- Kumar, N. M., Shimray, C. A., Indrani, D., & Manonmani, H. K., “Reduction of acrylamide formation in sweet bread with L-asparaginase treatment” Food and bioprocess technology, 7(3): 741-748.
- Gondal, A. H., Farooq, Q., Hussain, I., & Toor, M. D., “Role of Microbes in Plant Growth and Food Preservation” Agrinula: Jurnal Agroteknologi Dan Perkebunan, 4(2): 106-121.
- Thakur, N., Goyal, M., Sharma, S., & Kumar, D., “Proteases: Industrial applications and approaches used in strain improvement” 2018, In Biological Forum–An International Journal,10(1): 158-167.




















