SYNTHESIS OF NiO/ZnO NANOCOMPOSITES IN ETHYLENE GLYCOL
H. Tanzilla Hussain1, Bakhtawar Sajjad2, Umer Ali2, Auswa Nadeem2, Lubna Noor3 and Muhammad Akhyar Farrukh4
1Bahauddin Zakariya University, Multan, Pakistan
2University of Engineering and Technology, Lahore, Pakistan.
3University of Lahore, Pakistan
4Forman Christian College University, Lahore, Pakistan
*Correspondence | Bakhtawar Sajjad Email: bakhtawarsajjad898@yahoo.com
Citation | Hussain T, Sajjad B, Ali U, Nadeem A, Noor L and Farrukh M A “Synthesis of Nio/Zno Nanocomposites in Ethylene Glycol”. International Journal of Innovations in Science and Technology, Vol 02 Issue 01: pp 31-38. DOI: https://doi.org/10.33411/IJIST/2020020103
Received | January 03, 2020; Revised | February 14, 2020; Accepted | February 23, 2020; Published | March 04, 2020
___________________________________________________________________________
Abstract
The availability of water has become a serious concern globally, therefore, it is a need to use waste water by its treatment. This treated water can be used in various sectors e.g., agriculture, leather industry, textile industry and in chemical reactors etc. The oxides of semiconductor nanoparticles are effective catalyst which are used in wastewater treatment. Ethylene glycol is used in sol-gel method to synthesize NiO/ZnO nanocomposites. ZnCl2 along with NiCl2.6H2O were used as precursors during synthetization of NiO/ZnO nanocomposites. We used various techniques such as Thermogravimetric Analysis (TGA), Differential Scanning Calorimetry (DSC), Fourier Transform Infrared Spectroscopy (FTIR) and particle size analysis for synthesis of the nanocomposites which confirm that these nanocomposites act as catalyst.
KEYWORDS: Sol-gel method, Methylene blue, photo catalyst, Nano composites
Introduction
The availability of water has become a serious concern globally, because most of diseases are water borne. This concern leads the researchers to make proper treatment of wastewater. This treatment must be cost effective enough so that it can be used at large scale. The major benefit of recycled water is to save huge amount of pure water by reusing recycled water in agriculture, textile and in other industries. The oxides of semiconductor nanoparticles are effective catalyst which are used in wastewater treatment [1].
Zinc Oxide is n-type in nature which has a band gap of 3.2ev. ZnO has unique characteristics such as nontoxicity, photo catalytic activity, quantum efficiency, photosensitivity and the low cost [2]. It has micro-structural benefits due to which it is used in many devices e.g., gas sensors, Light Emitting Diodes (LEDs), Field Effect Transistors (FETs), Piezoelectric devices and the solar cells. Many researchers proved that ZnO is used as photo-catalytic for degradation of organic pollutants. Coupling of different semiconductors having variable energy levels have increased their functional properties as reported by many researchers [2, 3, 4, 5]. The integration two semiconductor oxides having different polarity such as n-type and p-type results in P-N junction and generates the effective electron pair.
Nickle oxide is p-type semiconductor having a band gap of 3.5-4.0ev. It has a number of applications in catalysis, chemical sensors, magnetic materials, electro chromic films, photovoltaic devices, and gas sensing instruments [3].
This study aims at synthesizing NiO/ZnO nanocomposites using ethylene glycol and involves the use of NiO/ZnO nanoparticles for photo-degradation of methylene blue in visible spectrum.
Materials and methods
Nickel chloride hexahydrate, sodium hydro oxide and methylene blue were acquired from sigma Aldrich, zinc chloride from Riedl-de Haen and Ethanol from labscan.
Preparation of NiO/ZnO nanocomposites
About 0.013g zinc chloride and 0.024g of nickel chloride hexahydrate was dissolved in 10 ml ethylene glycol. This solution was stirred for 20 minutes at room temperature to make it homogenous. About 20ml deionized water was added in 0.034g NaOH.
ZnCl2 and NiCl2.6H2O solution was shacked and the NaOH solution was added at 0.3ml/5 minutes up to the level when the pH of the solution was raised up to 9. This solution was centrifuged at 12500rmp up to 2 minutes. The precipitates were put in oven for the whole night and calcined at a temperature of 450oC up to 2 hours to get oxides out of hydroxides as expression below:
NiCl2.6H2O + ZnCl2 + 4NaOH → 4NaCl + Ni(OH)2 + Zn(OH)2 + 6H2O
Ni2+ + Zn2+ + 4OH- → Ni(OH)2 + Zn(OH)2
Figure 1 is showing the sequential flow of study to make nanocomposites of NiO/ZnO.
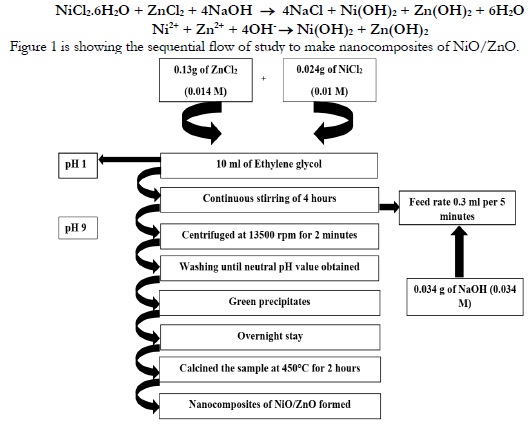
Figure 1. Scheme of NiO/ZnO Nanocomposite Preparation
Dye degradation
About 100ppm of Methylene blue solution was taken as stock solution. A 20ml was taken out of stock solution into volumetric flask and mixed with 80ml of distilled water. A 20ml of solution was put into the beaker from 20ppm solution and a quantity of 10mg NiO/ZnO nanocomposites were taken in the same beaker and stirred for 1 hour.
This solution was passed through ultraviolet visible spectrophotometer to examine the maximum wavelength of methylene blue. The absorption capacity was checked using ultraviolet visible spectrophotometer at 659nm for a duration of 15 minutes. Figure 2 is describing the step-by-step flow of dye degradation.
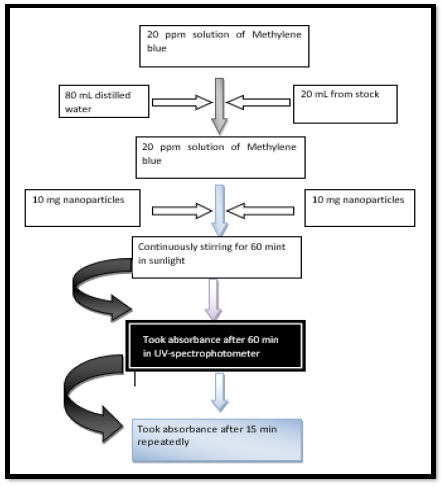
Figure 2. Degradation of Methylene blue using NiO/ZnO nanocomposites
Results and discussion
Thermo Gravimetric Analysis (TGA).
TGA analysis reveal that the curve moved steep till it became flat in shpae as shown in Figure 3. In thermogram, molecules of water which were adsorbed till it became horizontal at the temperature of 500 ºC. Water molecules which were adsorbed, then dehydrated below the temperature of 120 ºC. Overall loss was about 9% which was about 17.28 gmol-1. This change might be due to the loss of water molecules that were adsorbed upon the surface of nanocomposits.
The second loss which was observed at the temperature of 135 ºC to 230 ºC which was about 6% having a mass of about 11.52 gmol-1. This loss might be obtained by the disintegration of solvents and the residues of ash.
The third loss was observed between a temperature range 250ºC to 330ºC which was about 18%. It represented the changing of hydroxides to the oxides by losing two molecules of water. It was observed no change in weight beyond a temperature of 550ºC. This no change confirms the presence of ZnO nanoparticles. Same results were recorded by various researchers at various temperature ranges [4, 5, 6, 7, 8].
Differential Scanning Calorimetry (DSC).
The reference and the sample materials were exposed to a furnace and the changes were observed in both materials. We observed two peaks in DSC. The first peak represents the de-hydration of nickel hydro-oxide Ni (OH)2 at a temperature 270oC and the other one was observed at a temperature 420oC which is proof of existence of zinc hydro-oxide as shown in Figure 3.
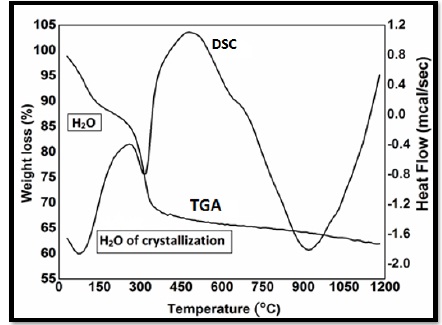
Figure 3. DSC curve of NiO/ZnO nanoparticles using Ethanol as a solvent
Particle size Analysis
The particles within a range of (1-100nm) are known as nanoparticles. Bt-90 was analyzer which used to determine the particle size. The particle size was estimated after 1 hour during synthesis. After one hour, the particle size was about 165nm having surface area 15.11m2/g. The particle size was computed as 135nm after two hours having surface area 19.32m2/g. After three and four hours the particle size was declined to 75nm to 55nm and the surface area was increased from 25.67m2/g to 37.21m2/g respectively as shown in table 1 and Figure 4.
Table 1. Particle size and surface area of nanoparticles.
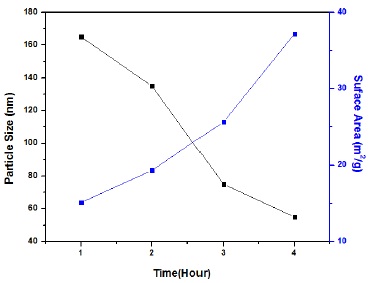
Figure 4. Relationship between Particle size and Specific surface area with respect to time.
Fourier Transform Infrared Spectroscopy (FTIS).
FTIR spectra was used to confirm the existence of functional groups in nanoparticles that generated a curve as showing in Figure 5. A dip was observed at 3475cm-1 which determine the existence of O-H functional group. Another dip was observed at 1631cm-1 which might be due to the movement of water molecules in curvature according to researches [9, 10, 11]. Another dip was observed at 1416cm-1 which show asymmetric stretching of C=O according to researches [12]. The dip at 638cm-1 shows the vibrations of metal oxygen metal bond. The dip formed at 475 cm-1 represents the stretching of ZnO nanoparticles, which also confirm the existence of NiO nanoparticles according to research [13].
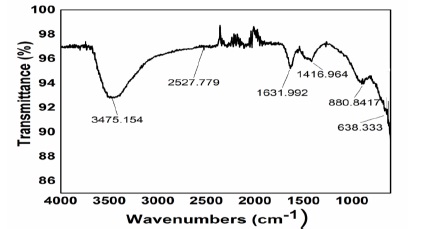
Figure 5 FTIR spectra of NiO/ZnO Nanocomposites
Degradation of Methylene Blue
Methylene blue degradation was checked through NiO/ZnO nanocomposites using spectrophotometer and a curve was formed in Figure 6. The process was executed in sunlight and the absorption rate was computed for a duration of 15 minutes. It was observed that the absorption rate decreased with passage of time due to decline of methylene blue concentration as shown in Table 2.
Table 2. Photocatalysis data of 20ppm methylene blue
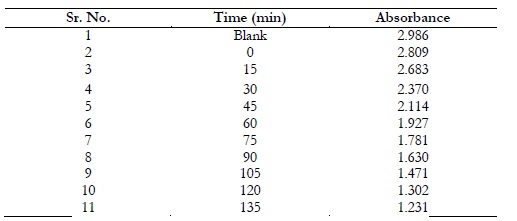
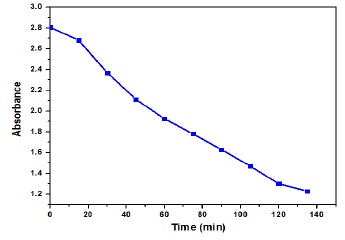
Figure 6. Methylene blue absorbance with respect to time.
Conclusion
Sol-gel technique is efficient to determine the preparation rate of NiO/ZnO Nano-composites. Various characterization techniques such as DSC, Particle size analyzer, UV-Vis and FTIR were performed. TGA analysis confirm the weight loss up to 18% which confirms the existence of hydroxide nanocomposites. Particle size was confirmed by particle size analyzer. Peak at 475 cm-1 might be due to stretching vibrations of the nanoparticles of ZnO. The catalysis activity checked by degradation of methylene blue in the presence of sun light. It was observed that the concentration of the methylene blue decreased as the time of reaction passed. It confirmed the catalytic activity of the NiO/ZnO nanocomposites.
Acknowledgement. H. Tanzilla Hussain, Bakhtawar Sajjad, Umer Ali and Auswa Nadeem are sincerely thankful to Government College University and Muhammad Akhyar Farrukh for conducting this research.
Author’s Contribution. All the authors contributed equally.
Conflict of interest. We declare no conflict of interest for publishing this manuscript in IJIST.
Project details. NIL
References
1. Alammar, T., Mudring, AV. Facile ultrasound-assisted synthesis of ZnO nanorods in an ionic liquid. Mater.Lett. 63: 732–735 (2009).
2. Zou, R., He, G., Xu, K., Liu, Q., Zhang Z., Hu J. ZnO nanorods on reduced graphene sheets with excellent field emission, gas sensor and photocatalytic properties. J. Mater. Chem. A. 1: 8445–8452 (2013).
3. Harraz, F.A., Mohamed, R.M., Shawky, A., Ibrahim, I.A. Composition and phase control of Ni/NiO nanoparticles for photocatalytic degradation of EDTA. J. Alloy Compds. 508: 133–140 (2010). 4. Perveen, H., Farrukh, M.A., Khaleeq-ur-Rahman, M. Synthesis, structural properties and catalytic activity of MgO-SnO2 nanocatalysts. Russ. J. Phys. Chem. 89: 99–107 (2015).
5. Sheena, P. A., Priyanka K. P., Aloysius Sabu, N., Sabu, B., Varghese, T. Effect of calcination temperature on the structural and optical properties of nickel oxide nanoparticles." Phys Chem Math. 5: 441-449 (2014).
6. Zak, A.K., W.H, Abd M., Darroudi, M., Yousefi, R. Synthesis and characterization of ZnO nanoparticles prepared in gelatin media. Mater.Lett. 65: 70-73 (2011).
7. Wang, Y., Zhu, J., Yang, X., Lu, L., Wang, X. Preparation of NiO nanoparticles and their catalytic activity in the thermal decomposition of ammonium perchlorate. Thermochimica Acta - THERMOCHIM ACTA. 437: 106-109 (2005).
8. Yang, X., Shao, C., Guan, H., Li, X., Gong, J. Preparation and Characterization of ZnO Nanofibers by Using Electrospun PVA/Zinc Acetate Composite Fiber as Precursor. INORG. CHEM. COMMUN. 7: 176-178 (2004).
9. Klančnik, G., Medved, J., Mrvar, P. Differential thermal analysis (DTA) and differential scanning calorimetry (DSC) as a method of material investigation. RMZ–Materials and Geoenvironment. 57(1): 127-142 (2010).
10. Pacholski, C., Kornowski, A., Weller, H. Self-assembly of ZnO: from nanodots to nanorods. Angewandte Chemie. 41(7):1188-1191 (2002).
11. Mohseni Meybodi, S., Hosseini, S.A., Rezaee, M., Sadrnezhaad, S.K., Mohammadyani, D. Synthesis of wide band gap nanocrystalline NiO powder via a sonochemical method." ULTRASON SONOCHEM. 19(4): 841-845 (2010).
12. Saad F., Oboudi, Nadir F., Habubi, Ghuson H., Mohamed, Sami S., Chiad. Composition and Optical Dispersion Characterization of Nanoparticles ZnO NiO Thin Films: Effect of Annealing Temperature. International Letters of Chemistry, Physics and Astronomy. 8(1): 78-86 (2012).
13. Dutta, S., Ganguly, B.N. Characterization of ZnO nanoparticles grown in presence of Folic acid template. J Nanobiotechnol. 10: 29 (2012).




















