Statistical Evaluation of Environmental Factors as Diabetogenic Agent in Type 2 Diabetes Mellitus
Saima Shokat1, Aasma Riaz2, Riffat Iqbal1, Atif Yaqub 1, Samreen Riaz 3
1Department of Zoology Government Collage University, Lahore
2College of Statistical and Actuarial Sciences University of the Punjab, Lahore
3Institute of Microbiology & Molecular Genetics University of the Punjab, Lahore
Corresponding author: *1 saimashokatgcu@gmail.com +923217523784
Citation | Shokat. S, Riaz. A, Iqbal. R, Yaqub. A, Riaz. S, “Statistical Evaluation of Environmental Factors as Diabetogenic Agent in Type 2 Diabetes Mellitus”. International Journal of Innovations in Science and Technology. Vol 4, Issue 1, 2022. Pp: 288-298.
Received | March 10, 2022; Revised | March 20, 2022 Accepted | April 01, 2022; Published | April 03, 2022.
The purpose of this study was to analyze the environmental factors affecting individuals with diabetes. A study was conducted among diabetes patients at the Lahore General Hospital's outdoor clinic. Data was collected using a standardized questionnaire after getting approval of patients being interviewed. SPSS 25.0 was utilized for analysis. Total 1000 people were chosen, 500 of whom were diabetic patients and the rest were non-diabetic. Environmental factors were investigated in a 1000-person research of diabetics and non-diabetics. To determine the relationship between patients with diabetes and environmental factors, the Chi-Square test and Mann-Whitney test were used to compare the effects of age, BMI, and sugar level fasting. The findings reveal that environmental factors play crucial effects on patients in term of age, BMI, and sugar level. I also used the odds ratio on diabetic and non-diabetic patients who have the Stroke, TIA, hypertension, and other environmental factors. The study revealed that diabetes is more persistent in industrial and urban region as 60% of the population living in these areas are under risk of diabetes. Moreover, the results showed that nearly 62% tap water consumers in rural areas were diabetic while 38% filtered water consumers in urban areas were diabetic. Smoking caused diabetes in nearly 22% people, 28% people suffered due to utilization of homeopathic medicines while 35% diabetic patients were found multivitamin consumers. Furthermore, the study depicted that among 1000 individuals under study, 56 % females were diabetic due to environmental factors. Diabetes has a direct relationship with the environment experienced by a patient.
Keywords: Diabetes; Environmental factors; Pakistan; Demographic variables; statistics.
|
ACKNOWLEDGEMENT: I am thankful to · Institute of Microbiology and Molecular Genetics, · University of the Punjab, Lahore,
|
· Department of Zoology, Government College University, Lahore, · College of Statistical and Actuarial Sciences University of the Punjab, Lahore,
|
CONFLICT OF INTEREST: The author(s) declare that the publication of this article has no conflict of interest. Project details. NA
|
INTRODUCTION.
Diabetes is a set of diseases marked by imbalance of insulin hormone. The pancreas (an organ beneath the stomach) normally releases insulin to help with the storage and utilization of sugars and fat from the diet. Diabetes develops when the pancreas fails to produce sufficient insulin, or the body fails to respond to insulin effectively [1]. The historic evidence of diabetes can be obtained from 1500 BC in Egyptian literature [2]. Elevated blood sugar is characterized by frequent urination, weight loss, increased hunger and increased thirst [3]. Acute consequences include hyperosmolar hyperglycemia diabetic ketoacidosis, and death. Long-term complications are caused by stroke, cardiovascular disease, chronic kidney illness, vision impairment and foot ulcers [4]. Type 1 diabetes is caused by insulin deficiency [5] while Type 2 diabetes is caused by a gradual decrease in insulin production [6]. Gestational diabetes is diagnosed in the second or third trimester of pregnancy [7]. Diabetes caused by other factors, such as syndromes e.g., neonatal diabetes and (MODY) i.e., maturity onset diabetes of the young, monogenic diabetes, pancreatitis, cystic fibrosis (exocrine pancreas disorders), drug induced diabetes or chemical is caused as a consequence of organ transplantation, or glucocorticoid use or AIDS /HIV treatment[8-10].
The prevalence of "stroke" is rising as a result of "macro vascular problems" while the ratio of coronary heart disease as compared to peripheral vascular disease is also rising.” Coronary diseases include thickening of the artery wall and cell translocation to the site of injury occur [11]. Micro vascular causes include nephropathy (kidney illness), neuropathy (nerve damage), and retinopathy (eye disease) [12]. Diabetic Neuropathy is a type of neuropathy caused by diabetes. Nerve damage affects about 60% of diabetic individuals, and it is a long-term condition linked to diabetes, Moreover Lethargy, numbness and discomfort are disorders can lead to leg cutting [13]. In Diabetic Nephropathy (DN) kidneys are affected by increased sugar level. In diabetic common foot disease, the feet are affected by the fungus infection and at the advance level, cutting of foot can happen [14].
Type 2 diabetes is a complex illness caused by a mix of hereditary and environmental risk factors. The etiopathogenesis of diabetes is influenced by environmental variables. Polluted air, soil, and water, as well as a bad diet, stress, lack of physical activity, stress, vitamin D insufficiency, enterovirus exposure, and immune cell destruction are all environmental factors leading to Type 2 diabetes [15], [16], it has close association with environmental factors. Dioxin, bisphenol A, herbicides, pesticides and the exposure with the industrial chemicals are the main environmental pollutants [17]. The interplay of environmental, psychosocial and biological factors is thought to be the cause of T2DM [18], [19]. Consequences of air pollution has been linked to altered endothelial function, inflammatory responses, and insulin resistance, as well as an increase in blood pressure[20], [21]. Although arsenic is one of the top 10 environmental toxins, there is conflicting evidence about its influence on type 2 diabetic mellitus and other human health effects. In Teharan, arsenic levels in the urine of newly diagnosed type 2 diabetes people are higher, and this is linked to smoking [22]. In Korean young adults, duration and amount of smoking has close association with the incidence of type 2 diabetes [23]. In the urban areas, people have high prevalence of type 2 diabetes as compared to rural areas and the male population is most affected [24]. Organochlorine pesticides is the risk factor for the type 2 diabetes mellitus [25]. Inorganic arsenic is associated in the prevalence of type 2 diabetes [18].
Environmental factors are the additional risk factors other than genetics and life style in development of diabetes moreover, the prevalence of insulin resistance affects the diabetes [26], [27]. The link between quality of life and psychiatric symptoms in diabetic patients with other chronic physical conditions and socio demographic factors was observed [28]. Endocrine disrupters for example arsenic, Zinc and cadmium interfere in glucose metabolism and act as diabetogenic agent. These endocrine disrupters harm the insulin sensitivity and beta cell function[29]–[38]. Rehman et al., 2021 explained the mechanism that is involved in association of arsenic with diabetes by dysfunction of pancreatic β-cell, disturbed insulin secretion and resistance [39]. While, Inorganic arsenic causes toxicity through polluted water and food consumption. Chronic arsenic exposure causes health effects so, it is very important to know about the metabolism of inorganic arsenic [40]. Researchers found that the insulin resistance of non-diabetic adults may associate the arsenic metabolism with rice consumption [41]. Heavy metals enter the human bodies and disrupt the metabolism of body and are evident of type 2 diabetes in developing countries. Heavy metals as pollutants have drastic health effects on human, woman and children health. It causes toxicity through food, air and drinking water. Demographic factors such as age, obesity, drinking water and life style effects the progression of type 2 diabetes [41]–[49]. The main objective of the study was to identify effect of the environmental factors on patients with type 2 diabetes.
METHODOLOGY.
Investigation site. Diabetic and Endocrine Metabolic Centre. Lahore General Hospital located at G, 152, 1 Canal Rd, Block G 1 Phase 1 Johar Town, Lahore, Punjab 54590
Population size. It was a cross-sectional study and convenient based sampling. The sample size of 1000 people was analyzed for type 2 diabetic and non-diabetic patients of general hospital Lahore, within a time duration of two months for this study i.e., 02-02-2021- 02-04-2021. The data was collected by face-to-face interview along with questionnaires.
Statistical Analysis. In order to find risk, Bivariate Analysis was applied. Reliability was tested by using Mann-Whitney test. The significance of association between each response and predictor, each variable was tested. Cronbach’s alpha was applied to measure internal consistency, which shows relationship between the set of items in a group. Cronbach’s alpha is a reliability scale which can be written as a function of the number of test items and the average inter-correlation among the items.

Conclusion was based on p-value. If p-value is less than α, then we reject the null hypothesis and conclude that there is significant difference between groups. (Daniel in “Applied Nonparametric Statistics”, 1978)
The association between an exposure and an outcome odds ratio was measured. Odd ratios were used to compare the relative odds of the occurrence of the outcome of interest (e.g., diabetes), given exposure to the variable of interest (e.g., environmental factors). The odds ratio can also be used to determine whether a particular exposure is a risk factor for a particular outcome, and to compare the magnitude of various risk factors for that outcome.
- OR=1 Exposure does not affect odds of outcome
- OR>1 Exposure associated with higher odds of outcome
RESULTS AND DISCUSSION.
This study consists of 1000 subjects in which both male and female were included. There were 500 diabetics and 500 non diabetic patients, different factors demographic (age, gender), environmental (smoking, residence area, regularity of medicine, multivitamins consumptions, eating fish, kind of water, kind of medicine and obesity/ BMI), biochemical (sugar fasting level) were determined and it was observed that complications have some association with these factors, significantly Diabetes Mellitus type 2 has association with environmental factors.
Descriptive analysis
The frequency and %ages of several demographic factors, environmental factor, biochemical factors and risk factors were examined in this section. There were 1000 individuals in this study. The results were debated based on frequency and %ages.
Demographic Factors
Table 1. Gender %age in overall population
|
Diabetic |
%age |
Gender |
%age |
|
DIABETIC NORMAL |
50.00% 50.00% |
Male |
44.00% |
|
Female |
56.00% |
The %age of the gender in 1000 respondents (diabetic 500 & non-diabetic 5000) which is 56% females and 44% males.
Table 2. %age of different age group
|
Variables |
Classification |
Diabetes |
Total |
||||
|
Male |
Female |
||||||
|
Count |
%age |
Count |
%age |
Count |
%age |
||
|
Age |
18-35 |
10 |
40 |
15 |
60 |
25 |
5.0 |
|
36-55 |
40 |
30.7 |
90 |
69.3 |
130 |
26.0 |
|
|
56-80 |
160 |
47.1 |
180 |
52.9 |
340 |
68.0 |
|
|
Above 80 |
5 |
|
|
5 |
1.0 |
||
Environmental factors
Table 3. Environmental Factors in type 2 diabetes (males and female)
|
%age of Environmental Factors determined into diabetic |
|||||||
|
|
Smoking |
Residence near industrial area |
Regularity Of Insulin In Patient |
Multivitamines |
Utilization of Homeopathic Medicine |
Fish consumption |
|
|
Diabetic |
Yes |
22.00% |
67.00% |
59.00% |
35.00% |
28.00% |
88.00% |
|
NO |
78.00% |
33.00% |
41.00% |
65.00% |
72.00% |
12.00% |
|
|
Non-Diabetic |
Yes |
20.00% |
60.00% |
0.00% |
26.00% |
80.00% |
78.00% |
|
NO |
80.00% |
40.00% |
0.00% |
74.00% |
20.00% |
22.00% |
|
Percentage of smoking, residence, Insulin regularity, multi vitamins consumption, taking Homeopathic medicine and fish consumption in 1000 respondents.
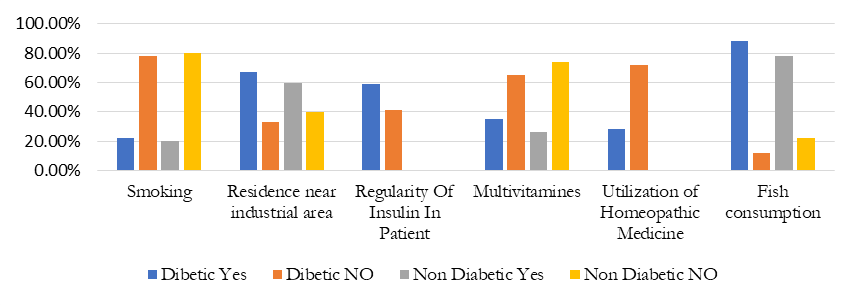
Figure 1. The effect different environmental factors on diabetic and non-diabetic
Figure 1 is showing the effect of various environmental factors and its comparison between diabetic and non-diabetic. High percentage of these environmental factors provide a correlation between environmental factors and increased number in type 2 diabetes.
Table 4. Environmental Factors in Diabetic Males and females
|
Residency |
water consumption |
Medications |
|||
|
Rural |
33.00% |
Tap |
62.00% |
Takes insulin |
15.00% |
|
Urban |
67.00% |
Filter |
38.00% |
Takes pills |
75.00% |
Various environmental factors were determined and their %age was recorded in table.
Table 5. BMI/Obesity of 1000 subjects (Males & Females)
|
Variables |
Classification |
Diabetes |
Total |
||||
|
Male |
Female |
||||||
|
Count |
%age |
Count |
%age |
Count |
%age |
||
|
BMI/ Obesity |
15-20 |
120 |
60 |
40 |
40 |
200 |
20.0 |
|
20-25 |
140 |
28 |
360 |
72 |
500 |
50.0 |
|
|
25-30 |
110 |
55 |
90 |
45 |
200 |
20.0 |
|
|
Above 30 |
60 |
60 |
40 |
40 |
100 |
10.0 |
|
BMI/Obesity of 1000 subjects ((Males & Females) categorized in different age groups and recorded.
Biochemical factors
Table 6. Biochemical factor Sugar Fasting Level in male & Female.
|
Variables |
Classification |
Diabetes |
Total |
||||
|
Male |
Female |
||||||
|
Count |
%age |
Count |
%age |
Count |
%age |
||
|
Sugar fasting level |
65-100 |
200 |
57.1 |
150 |
42.8 |
350 |
35.0 |
|
101-150 |
150 |
33.3 |
300 |
66.7 |
450 |
45.0 |
|
|
150-200 |
60 |
35.2 |
110 |
64.7 |
170 |
17.0 |
|
|
Above 200 |
20 |
66.6 |
10 |
33.3 |
30 |
3.0 |
|
1000 respondents divided into different age groups and Sugar Fasting Level is determined and recorded.
Risk Factors
Table 7. Duration of diabetes
|
Duration |
|
|
Non-Diabetic |
50% |
|
Less than 1 Year |
13.00% |
|
1 to 5 Years |
17.00% |
|
5 to 10 Years |
7.00% |
|
Greater than10 Years |
9.00% |
% age of duration of diabetes 50% are normal and other 50% are divided into different age groups.
Table 8. Risk factors due to Diabetes
|
|
Stroke |
TIA |
PeripShral Vascular Disease |
Hepatitis/Corona Virus/Cancer |
Other Major Surgeries Operations Etc |
Retinopathy |
Hypertention |
I.H.D Angina |
Myocardial Infection |
Congestive Cardiac Failure |
Cardiomyopathy |
Neuropathy |
Numbness/ Tingling In your Feet |
Stomach Problem |
||
|
Diabetic |
Yes |
25.0% |
27.0% |
48.0% |
0.00% |
87.0% |
36.00% |
41.0% |
69.0% |
30.0% |
35.0% |
29.0% |
32.0% |
34.0% |
23.0% |
|
|
NO |
75.0% |
73.0% |
52.0% |
100% |
13.0% |
64.00% |
59.0% |
31.0% |
70.0% |
65.0% |
71.0% |
68.0% |
65.0% |
77.0% |
||
|
Non-Diabetic |
Yes |
20.56% |
20.00% |
15.95% |
12.00% |
25.5% |
40.00% |
13.0% |
50.0% |
16.00% |
13.90% |
86.96% |
21.00% |
25.07% |
40.00% |
|
|
NO |
79.44% |
80.00% |
84.05% |
88.00% |
74.43% |
60.00% |
86.96% |
50.00% |
84.00% |
86.10% |
13.04% |
79.00% |
75.03% |
60.00% |
||
Various Risk Factors are more vulnerable in diabetic patients as compare to non-diabetic.
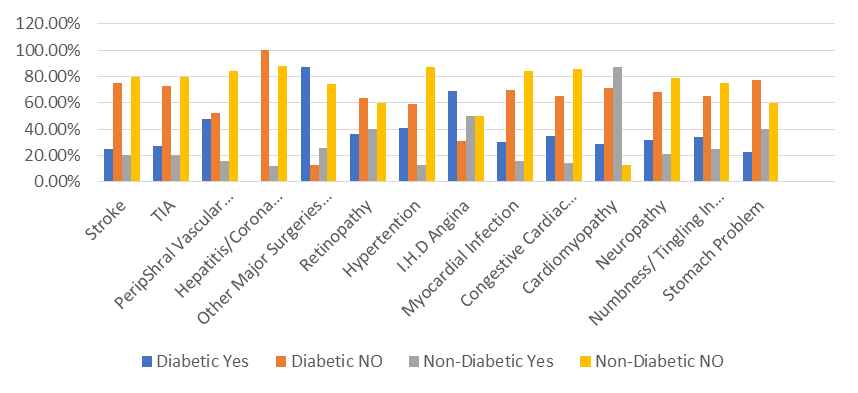
Figure 2. Comparison of different risk factors in diabetic and non-diabetic.
It shows that different levels of complication of various risk factors which are high in diabetic as compared to non-diabetic Diabetic
Analytical analysis
We analyzed the risk variable of diabetic and non-diabetic by using Bivariate Analysis
In this section association of variables was observed. Hence the significance of association between each predictor and response variable was tested by Pearson chi-square. When value of p was smaller than 0.05, then factors were significant (there is association), if p-value is greater than 0.05, then factors were insignificant (there was no association). Results of bivariate analysis for demographic variables, risk variables are presented in the tables given below:
Table 9. The Cronbach’s Alpha 0.705>0.5 indicates that our questionnaire is reliable for the data collection.
|
Reliability Statistics |
|
|
Cronbach's Alpha |
No of Items |
|
.705 |
30 |
In this section the dependent variable was diabetic and non-diabetic. And different demographic variable were independent variables.
Chi-square test
Table 10. Association of diabetic or non-diabetic with environmental factors.
|
No. |
Alternative Hypothesis |
χ2 |
P-value |
|
There is association between diabetic and non-diabetic and BMI |
15.862 |
0.001 |
|
|
There is association between diabetic or non-diabetic and fact of sugar fasting level. |
94.24 |
0.000 |
|
|
There is association between diabetic or non-diabetic and type of water used. |
10.336 |
0.001 |
|
|
There is association between diabetic or non-diabetic and residence (urban or rural). |
9.881 |
0.003 |
|
|
There is association between diabetic or non-diabetic and smoker. |
21.906 |
0.000 |
|
|
There is association between diabetic or non-diabetic and residence in industrial area. |
6.220 |
0.013 |
|
|
There is no association between diabetic or non-diabetic and stomach problem. |
1.019 |
0.313 |
|
|
There is no association between diabetic or non-diabetic and insulin. |
117.898 |
0.000 |
|
|
There is association between diabetic or non-diabetic and multi vitamins. |
19.525 |
0.000 |
|
|
There is association between diabetic or non-diabetic and homeopathic medicine. |
30.951 |
0.000 |
|
|
There is association between diabetic or non-diabetic and eat fish. |
10.001 |
0.004 |
|
|
There is association between diabetic or non-diabetic and numbness. |
9.929 |
0.003 |
|
|
There is association between diabetic or non-diabetic and skin allergy. |
75.661 |
0.000 |
Stomach problem have value above than 0.05 so, it has no association with diabetes while all other environmental factors have significant association with DM2.
In this section to check the normality of diabetic or non-diabetic score we use one sample Kolmogorov Simonov test
Table 11. To check the normality of diabetes score
|
No |
Alternative Hypothesis |
Kolmogorov Simonov test |
p-value |
|
Diabetic or non-diabetic are non- normally distributed. |
0.361 |
.000 |
The diabetic and non-diabetic were non-normal because their p-value was less than 0.05. So, to check the non-normal diabetic or non-diabetic effects on age, BMI and sugar fasting level. Use the Mann-Whitney U test.
Mann-Whitney u test:
In this section check the diabetic or non-diabetic effects on age, BMI and sugar fasting.
Table 12. Diabetic or Non-diabetic effects on age, BMI and sugar fasting.
|
No |
Alternative hypothesis |
Mann-Whitney U |
p-value |
|
Diabetic and non-diabetic persons have same effect on different age group |
893.500 |
0.003 |
|
|
The BMI level of diabetic and non-diabetic patients are same |
712.000 |
0.000 |
|
|
The sugar fasting level of diabetic and non-diabetic patients are same. |
187.000 |
0.000 |
The above table shows that the diabetic or non-diabetic affects people of all age groups, BMI and sugar fasting level
Table 13. Testing of gender with risk factor
|
Rick factor |
Odd ratios (diabetic or non-diabetic) |
95% Confidence interval |
|
|
Lower |
Upper |
||
|
Gender |
2.98 |
.870 |
3.980 |
|
Patient in family |
1.080 |
.511 |
2.282 |
|
Numbness |
1.401 |
0.705 |
2.784 |
|
Stroke |
0.151 |
0.053 |
0.428 |
|
Hypertension |
17.955 |
7.756 |
41.565 |
|
Skin allergy |
90.114 |
20.327 |
399.483 |
Odd ratio of diabetics or non-diabetic with respect to different risk factors
From the above odds ratio results, we can conclude that gender and stroke have low odds of occurrence of diabetes (as OR<1) whereas history of disease in family has almost no effect on the odds of occurrence of diabetes (as OR≈1). Meanwhile the odds of occurrence of diabetes because of Numbness, Hypertension and Skin allergy are higher (as OR>1) where skin allergy has the greatest odds with the value of OR being 141.923
The main goal of this study was to investigate diabetes-related environmental variables. A study was conducted in the city of Lahore to accomplish this goal, with data acquired from the General Hospital. The study's duration was set, and a convenient sampling procedure was applied. The information is gathered through a questionnaire. According to descriptive analysis the family history, residence in urban areas, obesity, smoking, living near industrial area play vital role in type 2 diabetes progressions. People were also affected with skin allergies. The association of type 2 diabetes and risk factors are investigated in Lahore district [50]. It is studied that the smoking is a key contributor in the development of type 2 diabetes [51]. According to this study, out of 1000 individual’s 56 % are females. The previous data correlated our findings that diabetes is more prevalent in urban areas and industrial areas so that environmental factors may play a role as diabetogenic agents [25], [52], [53]. According to previous study, arsenic is a diabetogenic agent which causes the skin allergy [54]–[56] and in our data 60 % people facing this problem because of consumption of polluted water. Out of 1000, 62 % people were tap water consumers. Statistical evaluation helps in proper analysis of data and know about its significance and to judge predictions.
CONCLUSION.
The findings of this practical based sampling produced information on the environmental variables of a general hospital-based study in Lahore, Pakistan, because the diabetes is most prevalent disease in Lahore. In this hospital mostly patients belong to middle class families which are mostly tap water consumers and are exposed to the environmental factors. Females were found to be more numerous than males. It's possible that the presence of more females than males is attributable to the population (Hospital) from whom the data was collected, resulting in a larger female-to-male ratio. In the overall analysis, the risk factors of kind of exercise, kidney problems, range of tests, and industry type are all highly associated with Type 2 Diabetes. The data was collected from respondents who lived near industrial areas, consuming polluted water and belong to low socioeconomic status, they were at more threat to type 2 diabetes. Environmental and risk factors have significant association with type 2 patients.
REFERENCES
[1] A. D. Association, “2. Classification and Diagnosis of Diabetes: Standards of Medical Care in Diabetes—2020,” Diabetes Care, vol. 43, no. Supplement_1, pp. S14–S31, Jan. 2020, doi: 10.2337/DC20-S002.
[2] B. C. R. LEUTHOLTZ, EXERCISE AND DISEASE MANAGEMENT. CRC Press, 2019.
[3] A. E. Kitabchi, G. E. Umpierrez, J. M. Miles, and J. N. Fisher, “Hyperglycemic Crises in Adult Patients With Diabetes,” Diabetes Care, vol. 32, no. 7, p. 1335, Jul. 2009, doi: 10.2337/DC09-9032.
[4] “Diabetes Home Remedies: How to Lower Blood Sugar Levels.” https://www.emedihealth.com/glands-hormones/diabetes/manage-diabetes (accessed Mar. 28, 2022).
[5] V. Anand et al., “Islet Autoimmunity and HLA Markers of Presymptomatic and Clinical Type 1 Diabetes: Joint Analyses of Prospective Cohort Studies in Finland, Germany, Sweden, and the U.S,” Diabetes Care, vol. 44, no. 10, pp. 2269–2276, Oct. 2021, doi: 10.2337/DC20-1836.
[6] “Chapter 17. Pancreatic Hormones and Diabetes Mellitus | Greenspan’s Basic & Clinical Endocrinology, 9e | AccessMedicine | McGraw Hill Medical.” https://accessmedicine.mhmedical.com/content.aspx?bookid=380§ionid=39744057 (accessed Mar. 28, 2022).
[7] P. J. Donovan and H. David Mcintyre, “Drugs for gestational diabetes,” Aust. Prescr., vol. 33, no. 5, pp. 141–144, 2010, doi: 10.18773/AUSTPRESCR.2010.066.
[8] R. Bonfanti et al., “Differences between transient neonatal diabetes mellitus subtypes can guide diagnosis and therapy,” Eur. J. Endocrinol., vol. 184, no. 4, pp. 575–585, Apr. 2021, doi: 10.1530/EJE-20-1030.
[9] “Diabetes: the tale of two types » HealthStyle.” https://www.healthstyle.net.au/article/diabetes-the-tale-of-two-types/ (accessed Mar. 29, 2022).
[10] A. D. Deshpande, M. Harris-Hayes, and M. Schootman, “Epidemiology of diabetes and diabetes-related complications,” Phys. Ther., vol. 88, no. 11, pp. 1254–1264, Nov. 2008, doi: 10.2522/PTJ.20080020.
[11] A. Ceriello, “Hyperglycaemia and the vessel wall: the pathophysiological aspects on the atherosclerotic burden in patients with diabetes,” Eur. J. Cardiovasc. Prev. Rehabil., vol. 17 Suppl 1, no. SUPPL. 1, May 2010, doi: 10.1097/01.HJR.0000368193.24732.66.
[12] J. Edwards and M. E. Beckman, “Methodological questions in studying consonant acquisition,” Clin. Linguist. Phon., vol. 22, no. 12, p. 937, 2008, doi: 10.1080/02699200802330223.
[13] C. M. Casellini and A. I. Vinik, “Clinical manifestations and current treatment options for diabetic neuropathies,” Endocr. Pract., vol. 13, no. 5, pp. 550–566, 2007, doi: 10.4158/EP.13.5.550.
[14] M. Eckhard, A. Lengler, J. Liersch, R. G. Bretzel, and P. Mayser, “Fungal foot infections in patients with diabetes mellitus--results of two independent investigations,” Mycoses, vol. 50 Suppl 2, no. SUPPL. 2, pp. 14–19, Sep. 2007, doi: 10.1111/J.1439-0507.2007.01425.X.
[15] R. Hashemi, M. Rahimlou, S. Baghdadian, and M. Manafi, “Investigating the effect of DASH diet on blood pressure of patients with type 2 diabetes and prehypertension: Randomized clinical trial,” Diabetes Metab. Syndr., vol. 13, no. 1, pp. 1–4, Jan. 2019, doi: 10.1016/J.DSX.2018.06.014.
[16] M. L. Larramendy and S. Soloneski, “Environmental Health Risk - Hazardous Factors to Living Species,” Environ. Heal. Risk - Hazard. Factors to Living Species, Jun. 2016, doi: 10.5772/61472.
[17] Y. Bi et al., “Advanced research on risk factors of type 2 diabetes,” Diabetes. Metab. Res. Rev., vol. 28 Suppl 2, no. SUPPL.2, pp. 32–39, Dec. 2012, doi: 10.1002/DMRR.2352.
[18] T. L. M. Hectors et al., “Environmental pollutants and type 2 diabetes: a review of mechanisms that can disrupt beta cell function,” Diabetologia, vol. 54, no. 6, pp. 1273–1290, Jun. 2011, doi: 10.1007/S00125-011-2109-5.
[19] S. Chatterjee, K. Khunti, and M. J. Davies, “Type 2 diabetes,” Lancet (London, England), vol. 389, no. 10085, pp. 2239–2251, Jun. 2017, doi: 10.1016/S0140-6736(17)30058-2.
[20] M. Schulz, M. Romppel, and G. Grande, “Built environment and health: a systematic review of studies in Germany,” J. Public Health (Oxf)., vol. 40, no. 1, pp. 8–15, Mar. 2018, doi: 10.1093/PUBMED/FDW141.
[21] S. Rajagopalan and R. D. Brook, “Air pollution and type 2 diabetes: mechanistic insights,” Diabetes, vol. 61, no. 12, pp. 3037–3045, Dec. 2012, doi: 10.2337/DB12-0190.
[22] A. Arab YarMohammadi, S. Arbabi Bidgoli, and P. Ziarati, “Increased urinary arsenic concentration in newly diagnosed type 2 diabetes mellitus: a gender-independent, smoking-dependent exposure biomarker in older adults in Tehran,” Environ. Sci. Pollut. Res. Int., vol. 28, no. 22, pp. 27769–27777, Jun. 2021, doi: 10.1007/S11356-020-10261-W.
[23] M. T. Boden and S. Gala, “Exploring correlates of diabetes-related stress among adults with Type 1 diabetes in the T1D exchange clinic registry,” Diabetes Res. Clin. Pract., vol. 138, pp. 211–219, Apr. 2018, doi: 10.1016/J.DIABRES.2017.10.012.
[24] Y. Huh, K. Han, M.-J. Choi, J. H. Kim, S. M. Kim, and G. E. Nam, “Association of Smoking Status With the Risk of Type 2 Diabetes Among Young Adults: A Nationwide Cohort Study in South Korea,” Nicotine Tob. Res., Feb. 2022, doi: 10.1093/NTR/NTAC044.
[25] S. A. Meo, I. Zia, I. A. Bukhari, and S. A. Arain, “Type 2 diabetes mellitus in Pakistan: Current prevalence and future forecast.,” J. Pak. Med. Assoc., vol. 66, no. 12, pp. 1637–1642, Dec. 2016, Accessed: Mar. 29, 2022. [Online]. Available: https://europepmc.org/article/med/27924966.
[26] K. Rangel-Moreno, B. Gamboa-Loira, L. López-Carrillo, and M. E. Cebrián, “Prevalence of type 2 diabetes mellitus in relation to arsenic exposure and metabolism in Mexican women,” Environ. Res., vol. 210, Jul. 2022, doi: 10.1016/J.ENVRES.2022.112948.
[27] J. R Barrera, “Insulin Resistance among Adults with Type 1 Diabetes Mellitus at the Philippine General Hospital,” J. Diabetes Metab., vol. 04, no. 10, pp. 1–6, 2013, doi: 10.4172/2155-6156.1000315.
[28] N. Ozdemir, A. Sahin, and A. Sahin, “Anxiety levels, quality of life and related socio-demographic factors in patients with type 2 diabetes,” Niger. J. Clin. Pract., vol. 23, no. 6, pp. 775–782, Jun. 2020, doi: 10.4103/NJCP.NJCP_523_19.
[29] S. Riaz, “Obesity as a Risk Factor for Diabetes Mellitus in the Local Population of Pakistan,” Univers. J. Clin. Med., vol. 2, no. 3, pp. 58–64, Nov. 2014, doi: 10.13189/UJCM.2014.020302.
[30] S. Riaz, “Evaluation and Analysis of Human Folate levels in Pakistani diabetic Population,” vol. 5, no. 12, pp. 1572–1576, 2014.
[31] S. Riaz, “Study of protein biomarkers of diabetes mellitus type 2 and therapy with vitamin B1,” J. Diabetes Res., vol. 2015, 2015, doi: 10.1155/2015/150176.
[32] S. Shahzad Alam, S. Riaz, and C. Borza, “Induction and Activities of Pyruvate Dehydrogenase and α-ketoglutarate Dehydrogenase in Type 2 Diabetic Patients and Therapy with Vitamin B1,” J. Adv. Med. Med. Res., vol. 12, no. 10, pp. 1–12, Jan. 2016, doi: 10.9734/BJMMR/2016/22530.
[33] M. T. Mursleen and S. Riaz, “Implication of homocysteine in diabetes and impact of folate and vitamin B12 in diabetic population,” Diabetes Metab. Syndr., vol. 11 Suppl 1, pp. S141–S146, Nov. 2017, doi: 10.1016/J.DSX.2016.12.023.
[34] S. Riaz, “Therapeutic Implication of Folate-Homocysteine Interaction in the Local Diabetic Pakistani Population,” vol. 2015, pp. 1–9, 2018.
[35] S. Riaz, “Analysis of Pyridoxine in the Male Diabetic Population of Lahore and Sheikhupura,” Ann. Diabetes, Metab. Disord. Control, vol. 2, no. 1, pp. 118–127, 2018.
[36] S. Riaz and M. Tariq, “Linkage of Micro Albuminuria and Serum Albumin Levels in the Diabetic Patients of Punjab University Premises,” vol. 2, no. 1, pp. 1–6, 2018.
[37] “Association of Serum Protein Levels in the Diabetic Patients with Risk of Cardiovascular Disease and Nephropathy in Pakistani Population.” https://www.scireslit.com/Diabetes/JRDM-ID21.php (accessed Mar. 29, 2022).
[38] Z. Joneidi, Y. Mortazavi, F. Memari, A. Roointan, B. Chahardouli, and S. Rostami, “The impact of genetic variation on metabolism of heavy metals: Genetic predisposition?,” Biomed. Pharmacother., vol. 113, May 2019, doi: 10.1016/J.BIOPHA.2019.108642.
[39] M. S. Rahaman et al., “Environmental arsenic exposure and its contribution to human diseases, toxicity mechanism and management,” Environ. Pollut., vol. 289, Nov. 2021, doi: 10.1016/J.ENVPOL.2021.117940.
[40] Y. Yang, L. Chi, Y. Lai, Y. C. Hsiao, H. Ru, and K. Lu, “The gut microbiome and arsenic-induced disease-iAs metabolism in mice,” Curr. Environ. Heal. reports, vol. 8, no. 2, pp. 89–97, Jun. 2021, doi: 10.1007/S40572-021-00305-9.
[41] X. Li, X. Wang, and S. K. Park, “Associations between rice consumption, arsenic metabolism, and insulin resistance in adults without diabetes,” Int. J. Hyg. Environ. Health, vol. 237, Aug. 2021, doi: 10.1016/J.IJHEH.2021.113834.
[42] Z. Rahman and V. P. Singh, “The relative impact of toxic heavy metals (THMs) (arsenic (As), cadmium (Cd), chromium (Cr)(VI), mercury (Hg), and lead (Pb)) on the total environment: an overview,” Environ. Monit. Assess., vol. 191, no. 7, Jul. 2019, doi: 10.1007/S10661-019-7528-7.
[43] S. Mishra et al., “Heavy Metal Contamination: An Alarming Threat to Environment and Human Health,” Environ. Biotechnol. Sustain. Futur., pp. 103–125, 2019, doi: 10.1007/978-981-10-7284-0_5.
[44] M. Al osman, F. Yang, and I. Y. Massey, “Exposure routes and health effects of heavy metals on children,” Biometals, vol. 32, no. 4, pp. 563–573, Aug. 2019, doi: 10.1007/S10534-019-00193-5.
[45] Z. Fu and S. Xi, “The effects of heavy metals on human metabolism,” Toxicol. Mech. Methods, vol. 30, no. 3, pp. 167–176, Mar. 2020, doi: 10.1080/15376516.2019.1701594.
[46] S. Tyagi, M. Siddarth, B. K. Mishra, B. D. Banerjee, A. J. Urfi, and S. V. Madhu, “High levels of organochlorine pesticides in drinking water as a risk factor for type 2 diabetes: A study in north India,” Environ. Pollut., vol. 271, Feb. 2021, doi: 10.1016/J.ENVPOL.2020.116287.
[47] M. Hendryx, J. Luo, C. Chojenta, and J. E. Byles, “Exposure to heavy metals from point pollution sources and risk of incident type 2 diabetes among women: a prospective cohort analysis,” Int. J. Environ. Health Res., vol. 31, no. 4, pp. 453–464, 2021, doi: 10.1080/09603123.2019.1668545.
[48] Y. L. Deng et al., “Associations between drinking water disinfection byproducts and menstrual cycle characteristics: A cross-sectional study among women attending an infertility clinic,” Int. J. Hyg. Environ. Health, vol. 241, p. 113931, Apr. 2022, doi: 10.1016/J.IJHEH.2022.113931.
[49] N. J. Pillon, R. J. F. Loos, S. M. Marshall, and J. R. Zierath, “Metabolic consequences of obesity and type 2 diabetes: Balancing genes and environment for personalized care,” Cell, vol. 184, no. 6, pp. 1530–1544, Mar. 2021, doi: 10.1016/J.CELL.2021.02.012.
[50] F. De la Fuente et al., “Green Space Exposure Association with Type 2 Diabetes Mellitus, Physical Activity, and Obesity: A Systematic Review,” Int. J. Environ. Res. Public Health, vol. 18, no. 1, pp. 1–18, Jan. 2020, doi: 10.3390/IJERPH18010097.
[51] M. Luciani, L. Montali, G. Nicolò, D. Fabrizi, S. Di Mauro, and D. Ausili, “Self-care is Renouncement, Routine, and Control: The Experience of Adults with Type 2 Diabetes Mellitus,” Clin. Nurs. Res., vol. 30, no. 6, pp. 892–900, Jul. 2021, doi: 10.1177/1054773820969540.
[52] M. Snobia, R. Samreen, A. Tooba, and R. Aasma, “Risk Factors Associated to Patients with Type 2 Diabetes in Lahore District,” Ann. Clin. Endocrinol. Metab., vol. 4, no. 1, pp. 011–019, May 2020, doi: 10.29328/JOURNAL.ACEM.1001014.
[53] M. H. Afridi et al., “Active Smokers and the risk of type 2 diabetes Mellitus in urban and rural area of kpk,” Am. J. Heal. Med. Nurs. Pract., vol. 7, no. 2, pp. 24–28, Jan. 2022, doi: 10.47672/AJHMN.915.
[54] T. Dendup, X. Feng, S. Clingan, and T. Astell-Burt, “Environmental Risk Factors for Developing Type 2 Diabetes Mellitus: A Systematic Review,” Int. J. Environ. Res. Public Health, vol. 15, no. 1, Jan. 2018, doi: 10.3390/IJERPH15010078.
[55] S. A. Meo, Y. A. Bin Muneif, N. A. Benomran, M. A. Alsadhan, R. F. Hashem, and A. S. Alobaisi, “Prevalence of Pre Diabetes and Type 2 Diabetes Mellitus among cement industry workers,” Pakistan J. Med. Sci., vol. 36, no. 2, p. 32, Jan. 2020, doi: 10.12669/PJMS.36.2.1266.
[56] A. Navas-Acien, E. K. Silbergeld, R. Pastor-Barriuso, and E. Guallar, “Arsenic exposure and prevalence of type 2 diabetes in US adults,” JAMA, vol. 300, no. 7, pp. 814–822, Aug. 2008, doi: 10.1001/JAMA.300.7.814.




















