Synthesis of NiO/ZnO Nanoparticles using 2-Propanol Solvent and their Applications for Methylene Blue Degradation
Lubna Noor1, Bakhtawar Sajjad2, Auswa Nadeem2, H. Tanzilla Hussain3, Shaista Ali4, Muhamad Akhyar Farrukh5,
1 University of Lahore, Pakistan
2 University of Engineering and Technology, Lahore, Pakistan.
3 Bahauddin Zakariya University, Multan, Pakistan
4 Government College University, Lahore, Pakistan
5 Forman Christian College University, Lahore, Pakistan
*Corresponding Author: Bakhtawar Sajjad bakhtawarsajjad898@yahoo.com
Citation | Noor L, Sajjad B, Nadeem A, Hussain H T, Ali S, and Farrukh M A “Synthesis of NiO/ZnO Nanoparticles using 2-Propanol Solvent and their Applications for Methylene Blue Degradation”. International Journal of Innovations in Science and Technology, Vol 02 Issue 01: pp 38-45.
DOI: https://doi.org/10.33411/IJIST/2020020104
Received | January 03, 2020; Revised | February 13, 2020; Accepted | February 25, 2020; Published | March 03, 2020
___________________________________________________________________________
Abstract.
The nanoparticles play a significant role in fabrication process which are used at large scale in various fields e.g., sensors, electronics drug delivery, optics, catalysis and in water purification process. Nanoparticles (NiO/ZnO) were synthesized using sol-gel technique. In this method, 2-propanol was taken to analyze the particle size. Fourier Transform Infrared Spectroscopy (FT-IR) confirmed the presence of ZnO/NiO. Ultraviolet Visible (UV) data recorded a band gap for ZnO that was 4.1 eV while UV spectrum of methylene blue demonstrated a decrease in concentration of methylene blue while using NiO/ ZnO as catalyst.
Keywords: Sol-gel, Nanocomposites, Methylene blue, Photocatalyst
Introduction
The existence of nanoparticles in metal oxides are significant while performing fabrication which leads to exchanging their bulk properties. These nanoparticles are widely used in various fields e.g., sensors, electronics drug delivery, optics, catalysis and in water purification process [1]. These nanoparticles have very diverse properties as insulator, metal and semi-conductor which are used in piezoelectric devices, microelectronic circuits and the gas sensors [2]. Zinc oxide are in extraordinary in nature as these have low cost, highly photosensitive and non-toxic. These are used in solar cells, light emitting diodes, photonic crystals, acoustic wave filters, modulator wave guides, transparent conductors and photo detectors. These nanoparticles are unique due to their properties such as they have a band gap of 3.37ev having energy as 60mev [3-8]. The nanoparticles of nickel oxide act as cathode in superconductors [9], because these are super paramagnetic and ferromagnetic in behavior [10]. NiO has a band gap 3.5 eV which behave like p-type semiconductors and applied in various fields such as battery manufacturing, electro chromic film, and gas sensors [11-15]. NiO has been investigated extensively due to its structure. The electrons of NiO are spread over a large range of energy due to strong coulomb repulsion [11, 16].
ZnO based nanocomposites are interesting for photocatalytic degradation because various advantages including direct band gap, anisotropic growth, high electron mobility and simple controlling of its morphology [17]. Montini and co-authors synthesized NiO/ZnO nanocomposite and investigated photocatalytic properties of nanoparticles [18].
The aim of this research is to synthesize NiO/ZnO nanoparticles by using 2 propanol as solvent and the application of these nanoparticles as catalyst for methylene blue dye degradation i.e. a harmful dye.
Material and Methods:
NiCl2. 6H2O, Methylene blue and 2-propanol was acquired from sigma Aldrich and ZnCl2 was from Riedl-de-Haen. ZnCl2 was taken 0.013g and NiCl2. 6H2O was taken 0.024g. Both were dissolved in 10 ml 2-propanol. The pH of this solution was measured as 1. The solution was stirred for 5-minutes at room temperature and raised its pH up to 9. Now this solution was centrifuged at 1300rmp for 2-miniutes. To get dry precipitates, we put these in oven overnight and then calcined for two hours at 450oC to obtain oxides only. The chemical reaction is as below:
NiCl2.6H2O + ZnCl2 + 4NaOH → 4NaCl + Ni (OH)2 + Zn (OH)2 + 6H2O
Ni2+ + Zn2+ + 4OH- → Ni (OH)2 + Zn (OH)2
Figure 1 is showing the flow of study adopted in this research to get NiO/ZnO nanoparticles.
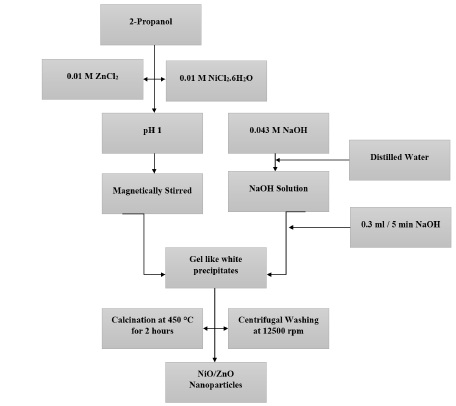
Figure 1. Systematic Synthesis of NiO/ZnO
NiO and ZnO nanoparticles were investigated for photo catalytic activity. A solution of Methylene blue was prepared in 20ppm and passed through ultraviolet spectrometer. The wavelength of this solution was recorded as 659nm. About 20ml of this solution was taken into a separate beaker and 10mg of NiO/ZnO nanoparticles were added in it. This solution was further passed through ultraviolet spectrometer at a wavelength of 659nm and the catalytic activity of NiO/ZnO was measured.
Results and discussion
Fourier Transmission Infrared Spectroscopy (FT-IR)
FTIR spectrum of NiO/ZnO nanocomposites was recorded after calcination. Figure 2 is showing the percentage of transmittance at various wavenumbers. The broad absorption band was recorded at a wavenumber of about 3350 cm−1 which is attributed to O–H stretching vibrations. The band number at 1631 cm−1 is attributed to bending mode (H–O–H). The peak at band number 1416 cm−1 is due to symmetric stretching of C=O. A peak was recorded around 880.8417 cm-1 which was due to metal oxygen bond while the band number at 637.321 cm-1 represents the stretching vibrations of Ni-O-H bond. The peak at band number 470.201 cm-1 represents the peak of Ni-O nanoparticles. The absorption band at 470.201 cm-1 describes the stretching mode of ZnO which lie between 450 cm-1 to 500 cm-1 The same results were recorded were reported by various researchers [17,18,19,20,21,22,23].
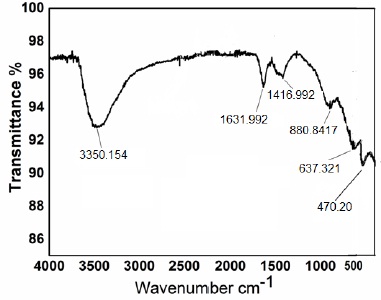
Figure 2. FT-IR of calcined NiO/ZnO
Particle Size Analysis
The particle size of nickel oxide and zinc oxide nanoparticle were measured by taking Bt-90 analyzer. The results reported that the surface area and the particle size are in inverse relation to each other. Particle size was estimated as 175nm, 139nm, 92nm and 69nm after 1, 2, 3 and 4 hours respectively as showing in Table 1 and Figure 3.
Table 1. Relationship between particle size and the surface area of NiO/ZnO using 2-Propanol as solvent.
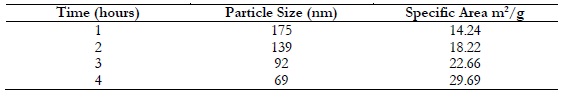
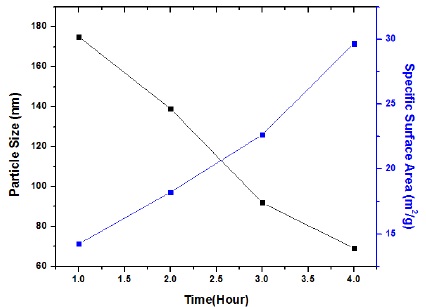
Figure 3 Particle size, time and specific surface area if NiO/ZnO nanoparticles using 2-propanol as solvent
UV- Visible Spectroscopy:
Band gap of NiO and ZnO nanoparticles was measured using the following equation:
(hνα)n = A (hν-Eg) Eq (1)
In equation (1) “A” indicates the absorption, “Eg” is band gap, “hν” determines the photo energy and “α” represents the absorption coefficient. The value of n is taken as 2 in case of direct transition and ½ for indirect transition.
The band gap of NiO/ZnO was estimated as 4.1ev. Figure 4 is showing the ultraviolet and visible spectrum of NiO/ZnO nanoparticles.
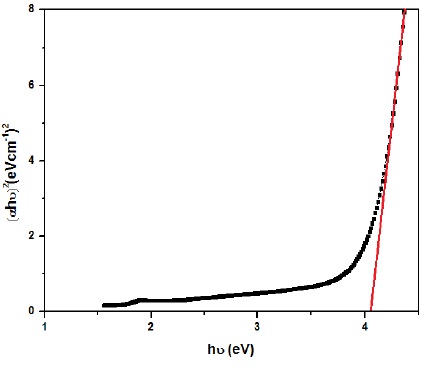
Figure 4. the band gap of NiO/ZnO.
Photocatalytic Activity of NiO/ZnO nanoparticles:
The photocatalytic activity of NiO/ZnO nanoparticles was estimated using UV-visible spectrophotometer with methylene blue. The absorption capacity of the solution was estimated by exposing it to sunlight and the absorption was estimated after every 15 minutes. The absorption capacity was declined with passage of time as shown in Table 2.
Table 2. Photocatalysis data of methylene blue
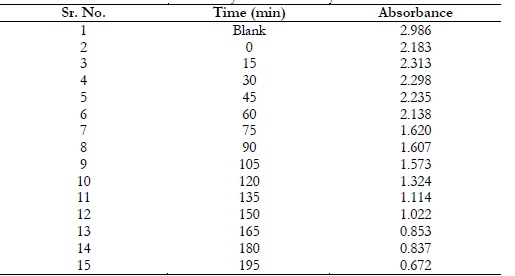
Figure 5, 6 and 7 are clearly representing the catalytic nature of NiO/ZnO nanoparticles worked. Figure 5 is showing the decrease in absorbance with the passage of time confirming the decreasing concentration of methylene blue in the presence of nanoparticles.
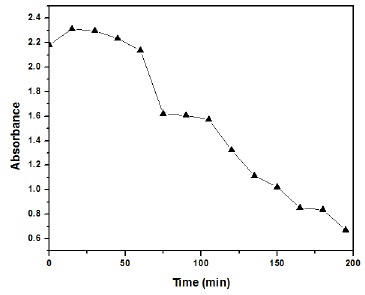
Figure 5 Methylene Blue absorbance with respect to time.
Figure 6 is showing that Methylene Blue was degraded with passage of time.
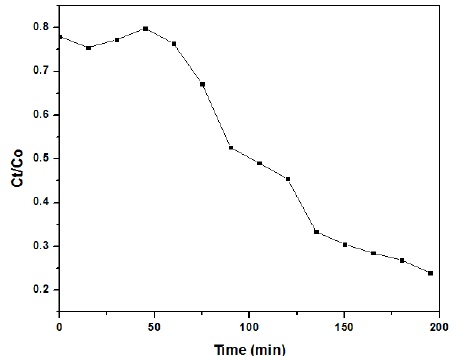
Figure 6 Degradation of MB dye as a function of UV light irradiation time
Figure 7 confirmed that NiO/ZnO nanoparticles degraded the dye up to 75% with passage of time.
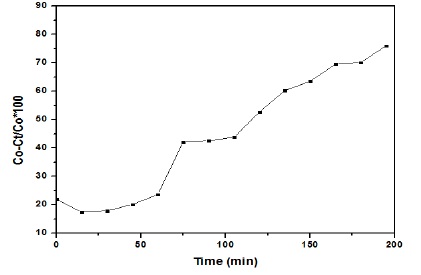
Figure 7. MB degradation efficiency of ZnO NPs
Conclusion
Nickel oxide and zinc oxide nanoparticles were synthesized using sol-gel method, and the particle size was measured as 69nm and 52.1nm of uncalcined and calcined nanoparticles respectively. A band gap of 4.1ev was measured for NiO and ZnO nanoparticles. Photocatalytic activity of nickel oxide and zinc oxide nanoparticles showed maximum degradation up to 75% under sunlight irradiations. Nickel oxide and zinc oxide nanoparticles can be used as active catalyst for methylene blue degradation.
Acknowledgement. Lubna Noor, Bakhtawar Sajjad, H.Tanzilla Hussain and Auswa Nadeem are sincerely thankful to Government College University, Muhammad Akhyar Farrukh and Shaista Ali
for conducting this research.
Author’s Contribution. All the authors contributed equally.
Conflict of interest. We declare no conflict of interest for publishing this manuscript in IJIST.
Project details. NIL
References
1. Maynard, Andrew & Aitken, Robert & Butz, Tilman & Colvin, Vicki & Donaldson, Ken & Oberdörster, Günter & Philbert, Martin & Ryan, John & Seaton, Anthony & Stone, Vicki & Tinkle, Sally & Tran, Lang & Walker, Nigel & Warheit, David. Safe handling of nanotechnology. Nature. 444: 267-9 (2006).
2. Zhang, F., Chan, S.W., Spanier, J., Apak, E., Jin, Q., Robinson, R., Herman, I. Cerium oxide nanoparticles: size-selective formation and structure analysis. Appl. Phys. Lett. 80(1): 127-129 (2002).
3. Piva, D.H., Piva, R.H., Rocha, M.C., Dias, J.A., Montedo O.R.K., Malavazi, I., Morelli, M.R. Antibacterial and photocatalytic activity of ZnO nanoparticles from Zn(OH)2 dehydrated by azeotropic distillation, freeze drying, and ethanol washing. Advanced Powder Technology 28:463 (2017).
4. Sood, S., Kumar, A., Sharma, N. Photocatalytic and Antibacterial Activity Studies of ZnO Nanoparticles Synthesized by Thermal Decomposition of Mechanochemically Processed Oxalate Precursor. Chemistry select. 1: 2016, 6925.
5. Kavitha, T., Iyengar, A., Gopalan, llLee, K.P., Park, S.Y. Glucose sensing, photocatalytic and antibacterial properties of graphene–ZnO nanoparticle hybrids Carbon. 50: 2994 (2012).
6. Elavarasan, N., Kokila, K., Inbasekar, G., Sujatha, V. Evaluation of photocatalytic activity, antibacterial and cytotoxic effects of green synthesized ZnO nanoparticles by Sechium edule leaf extract. Research on Chemical Intermediates. 43: 3361 (2017).
7. Pradeev Raj, K., Sadaiyandi, K., Kennedy, A., Sagadevan, S. Photocatalytic and antibacterial studies of indium-doped ZnO nanoparticles synthesized by co-precipitation technique. Journal of Materials Science: Materials in Electronics. 28: 19025 (2017).
8. Reddy Yadav, L.S., kumar, D., Kavitha, C., Rajanaika, H., Daruka Prasad, B. Antibacterial and Photocatalytic activities of ZnO Nanoparticles : Synthesized using Water Milon Juice as Fuel. International Journal on Nanoscience. 15: 1 (2016).
9. Wang, D.W., Li, F., Cheng, H.M. Hierarchical porous nickel oxide and carbon as electrode materials for asymmetric supercapacitor. Journal of Power Sources 185(2): 1563-1568 (2008).
10. Ichiyanagi, Y., Wakabayashi, N., Yamazaki, J., Yamada, S., Kimishima, Y., Komatsu, E., Tajima, H. Magnetic properties of NiO nanoparticles. Physica B:Condensed Matter 329: 862-863 (2003).
11. Park, Y.R., Kim, K.J. Sol-gel preparation and optical characterization of NiO and Ni1-xZnxO thin films. Journal of Crystal Growth 258: 380-384 (2003).
12. Curri, M.L., Agostiano, A., Mavelli, F., Della Monica, M. Reverse micellar systems: self organised assembly as effective route for the synthesis of colloidal semiconductor nanocrystals. Materials Science and Engineering 22: 423-426 (2002).
13. Yoshio, M., Todorov, Y., Yamato, K., Noguchi, H., Itoh, J.I., Okada, M. Mouri, T. Preparation of LiyMnxNi1-xO2 as cathode for lithium-ion batteries. Journal of Power Sources 74(1): 46-53 (1998).
14. Yang, H.X., Dong, Q.F., Hu, X.H., Ai, X.P., Li, S.X. Preparation and characterization of LiNiO2 synthesized from Ni(OH)2 and LiOH.H2O . Journal of Power Sources 79(2): 256-261(1999).
15. Miller, E.L., Rocheleau, R.E. Electrochemical Behavior of Reactivity Sputtered Iron-Doped Nickel Oxide . Journal of Electrochemical Society 144(9): 3072-3077 (1997).
16. Bengone, O., Alouani, M., Ch,l P., Hugel, J. Implementation of the projector augmented-wave LDA+U method: Application to the electronic structure of NiO. Phys. Rev. B: Condens. Matter Mater. Phys., 62: (2000).
17. Kovalenko, A.A., Baranov, A.N., Panin, G.N. Synthesis of ZnO/NiO nanocomposites from ethanol solutions. Russ. J. Inorg. Chem., 53(10): 1546–1551 (2008)
18. Hameed, A., Montini, T., Gombac, V., Fornasiero, P. Photocatalytic decolourization of dyes on NiO-ZnO nano-composites. Photochem. Photobiol.Sci., 8(5): 677-682 (2009).
19. Zak, A.K., W.H, Abd M., Darroudi, M., Yousefi, R. Synthesis and characterization of ZnO nanoparticles prepared in gelatin media. Mater.Lett. 65: 70-73 (2011).
20. Meybodi, S., Mohseni, Hosseini, S.A., Rezaee, M., Sadrnezhaad, S.K., Mohammadyani, D. Synthesis of wide band gap nanocrystalline NiO powder via a sonochemical method. Ultrasonics sonochemistry, 19(4): 841-845 (2012).
21. Saad, F., Oboudi, Nadir, F., Habubi, Ghuson, H., Mohamed, Sami, S.,Chiad. Composition and Optiocal Disprsion Characterization of Nanoparticles ZnO NiO Thin Films: Effect of Annealing Temperature. International Letters of Chemistry, Physics and Astronomy, 8(1): 78-86 (2012).
22. Alagiri, M., Ponnusamy, S., Muthamizhchelvan, C. Synthesis and characterization of NiO nanoparticles by sol–gel method. Journal of Materials Science: Materials in Electronics, 23(3): 728-732 (2012). 23. Dutta, S., Ganguly, B.N. Characterization of ZnO nanoparticles grown in presence of Folic acid template. J Nanobiotechnol. 10: 29 (2012).




















