Synthesis of Picric Acid at Domestic Scales.
Afshan Saleem1, Noreen Rafi1, Sumara Qasim1, Usama Ashraf2, Nasir Hussain Virk2
1Department of Chemistry, University of Engineering & Technology Lahore.
2COMSATS University Islamabad, Lahore Campus
*Correspondence | Afshan Saleem E.Mail. afshan.jutt66@yahoo.com Department of
Chemistry, University of Engineering & Technology Lahore.
Citation | Saleem.A1, Rafi. N1, Qasim.S1, Ashraf.U1, Virk.N.H1”. Synthesis of Picric Acid at Domestic Scales.International Journal of Innovations in Science and Technology, Vol 01 Issue 02: pp 62-78, 2019.
DOI | https://doi.org/10.33411/IJIST/2019010202
Received | Feb15, 2019; Revised |March20, 2019 Accepted | March24, 2019; Published | March28, 2019.
_____________________________________________________________________
Abstract.
Picric acid and its derivatives are widely used in various applications/industries. The first synthetic dye was prepared in 1771 using picric acid. It was used to dye silk fabric into greenish-yellow color. In this study, Picric acid and their derivatives were synthesized and characterized by their physical and chemical properties. The derivatives of Picric acid which are considered in this research include Picramic acid and Sodium Picramate. The physical properties like melting point, colors, physical state and solubility of Picric acid and its derivatives were determined and confirmed using IR Spectra. IR spectra proved efficient in scanning and mapping.
Keywords: Picric acid, Synthetic dye, Picramic acid, Sodium picramate and IR Spectra.
Introduction.
The word Picric was derived from Greek language which mean a bitter acid that reflect harshness in taste. Picric acid is commonly known as pollutant which is extremely dangerous for human body like trinitrotoluene [1]. Trinitrophenol is commonly known as Picric Acid.Trinitrophenol is extensively used in many industries such as fuel cells, leathers, pharmaceuticals, explosive, agriculture and polymer etc. Picric acid is highly dangerous for liver, eyes, kidney and the respiratory system [2,3]. Picric acid was first used to compute the glucose level in blood in start of 20th century. When picric acid, sodium carbonate, and glucose are mixed and heated, a red color specie is formed which is useful to measure the glucose level in human body. Picric acid in wet form is used to dye skin that interact with protein available in skin for making it dark brown which remain brown till one month [4]. Picric acid is extensively used in pharmaceutical industry where it is stocked as antiseptic and used for the treatment of smallpox, malaria, herpes, and burns. It belongs to a family of highly acidic phenols [1]. The structure and characteristics of picric acid are shown in the figure below,

Figure 1: Structure of picric acid
Chemical formula: C6H3N3O7
Chemical name: 2, 4, 6-trinitrophenol
Appearance: colorless to yellow solid
Molecular weight: 229.10 g/mol
Melting point: 121-1230C
Boiling point: 3000C
Density: 7.9
The most common use of Picric Acid is in dyeing leather, silk and wool. This is considered the oldest dying method, which was applied by woulfe first in 1771, which is called nitro dyeing. This way of dying is used for transformation of designs on fabrics woven by polyester [2,3]. It is also used in data recording in optical wavelength ranges inkjet printing and thermal printing. Picric Acid is mixed with alkyd resin commonly
known as electrophoretic coating of Aluminum. Phenol is sulphonated to form both o-phenolsulphonic and p-phenolsulphonic acid. Both o-phenolsulphonic and p-phenolsulphonic acid go through nitration to form 2, 4, 6-trinitrophenol but they each take a slightly different path [4]. This process is show in Figure 2.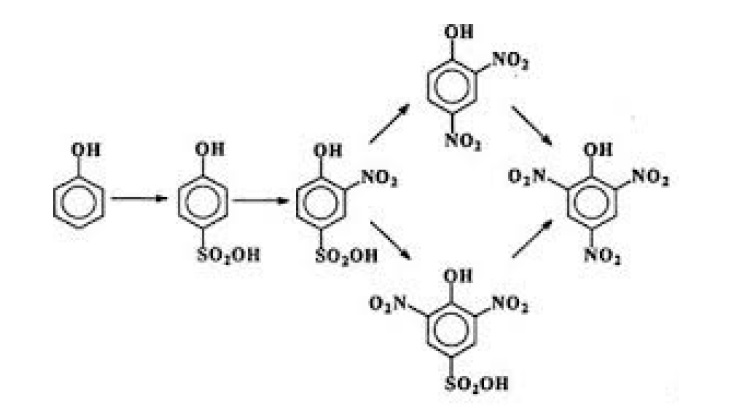
Figure 2. Preparation of picric acid.
PICRAMIC ACID
2-amino 4,6 dinitrophenol is known as Picramic Acid. Its color is dark red that is insoluble in water. Its nature is explosive in dry state [5,6,7]. It ignites in open air and explodes readily. Picramic acid is highly flammable which can be dissolved in acetic acid, chloroform, water and alcohol and most of other solvents. The alcoholic picric acid is neutralized with Aluminum hydroxide to obtain picramic acid. Finally, this solution is added in Hydrogen Sulphide to get red crystals of picramic acid. Picramic acid is toxic, explosive and bitter in taste. The structure of picramic acid is shown in Figure 2.
Figure 2. Preparation of picric acid.
PICRAMIC ACID
2-amino 4,6 dinitrophenol is known as Picramic Acid. Its color is dark red that is insoluble in water. Its nature is explosive in dry state [5,6,7]. It ignites in open air and explodes readily. Picramic acid is highly flammable which can be dissolved in acetic acid, chloroform, water and alcohol and most of other solvents. The alcoholic picric acid is neutralized with Aluminum hydroxide to obtain picramic acid. Finally, this solution is added in Hydrogen Sulphide to get red crystals of picramic acid. Picramic acid is toxic, explosive and bitter in taste. The structure of picramic acid is shown in Figure 2.
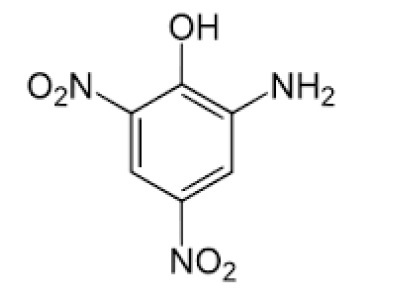
Figure 3. Structure of picramic acid
Chemical formula: C6H5N3O5
Chemical name: 2-amino-4, 6-dinitrophenol
Molecular mass: 199.12g/mol
Appearance: Brown paste
Density: 1.74 g mol-1
Melting point: 1690C
Boiling point: 386.30C
Flash point: 187.50C
SODIUM PICRAMATE
The salt of picramic acid is called sodium picramate which is a phenolic compound [8,9,10,11]. Both the picramic acid and sodium picramate are used in dying hair color which are available in market commercially. The addition of picramic acid to hair dye formula, produces a salt known as sodium picramate. The cosmetic review panel suggested that an ingredient would be applicable to another ingredient. A caution statement determines of the amount of picramic acid to be added in cosmetic ingredients which are useable by hums to get rid of skin irritation [12,13,14]. The structure of picramic acid is shown below.
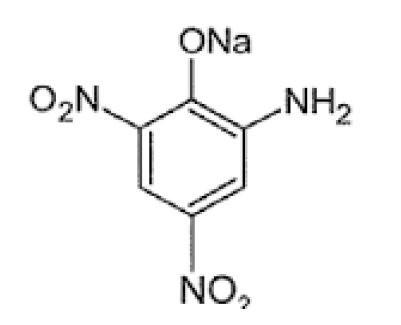
Figure 4. Structure of sodium picramate
Chemical formula: C6H4N3NaO5
Chemical name: Phenol, 2-amino-4, 6-dinitro-, sodium salt
Boiling point: 386.30C at 760 mmHg
Molecular weight: 221.10
Flash point: 187.50C
Appearance: Dark red prescak
Melting point: 98.80C (Decomposition)
Dinitrophenols were used as insecticides herbicides, acaricides and fungicides due to their biocidal activity [15]. The dinitrophenol are toxic to insects, plants, humans and animals therefore these are avoided to be used for insecticides anymore.The chemical and thermolytic treatments are avoided for removal of nitrophenols due to their unfriendly behaviors in context of environment [16]. This strategy is sustainable to reduce the cost of reduction of nitrophenol for all e.g., contaminated, waste and ground water. Sodium picramate and picramic acid are used to change the color of hair permanently. The shades in hair colors are dependent upon the composition and proportion of ingredients used in composition of final product [17,18,19]. The proportion of ingredients is amalgamated in a way that they interact in highly controlled processes.
3. Material and methods:
Apparatus:
250 mL glass beaker
Pipette
Stirring Rod
Funnel
Filter paper
Ice bath
3.2 Chemicals Required:
98% Conc. H2SO4
68% Conc. HNO3
Phenol
Ethanol
SYNTHESIS OF PICRIC ACID
Preparation of Solutions:
Preparation of 10 milimole of Phenol:
1 mole of Phenol = 94.11 g (1 mole = 1000 milimoles)
1000 milimole = 94.11
1 milimole = 94.11/1000 = 0.09411 g
10 milimole = 0.09411x10 = 0.9411 g
Preparation of 10 milimole of HNO3:
1 mole of HNO3 = 63.01 g (1 mole = 1000 milimoles)
1000 milimole = 63.01 g
1 milimole = 63.01/1000 = 0.06301 g
10 milimole = 0.06301x10 = 0.6301 g
Preparation of 10 milimole of H2SO4:
1 mole of H2SO4 = 98.08 g (1mole=1000 milimoles)
1000 milimole = 98.08 g
1 milimole = 98.08/1000 = 0.09808 g
10 milimole = 0.09808x10 = 0.9808 g
Procedure
Powdered phenol (0.941 g) were weighted and added to a 250 mLbeaker containing concentrated sulfuric acid (0.980 g) [20]. Powdered phenol (0.941 g) were weighted and added to a 250 ml beaker containing concentrated sulfuric acid (0.980 g). The mixture was shakened properly and was heated for half an hour for at 100℃ and its color was turned to dark color. We obtained a tube, added chilled water at -5 ℃ and put
the mixture in this cold water [21,22,23]. The mixture became a viscous syrup which was put in a fume cupboard by addition of 47 ML of cold nitric acid. It was important to maintain the temperature of the mixture at -5 °C before addition of the acid. A vigorous reaction was occurred that produced a red colored gas which was examined and recognized as nitrogen dioxide. The whole mixture was heated for 90 minutes with
random shaking [24,25]. Finally, the mixture became normalized at room temperature and we added 313 ml of cold water that turned the picric acid in to crystals. We used distilled water to filter out picric acid and for removal of taraces of nitric acid. We obtained the yellowish crystalline mass which was 40 grams. We obtained 2 volumes of water and 1 volume of ethanol for recrystallization of picric acid. Recrystallization is important to obtain purified picric acid [26,27]. It was observed that 9ml solvent (water + ethanol) is required to purify 1 gram of picric acid.
A vacuumed filter was used to remove crystals and these crystals were dried using dissector at 118 . Now we calculated the yield of picric acid. Almost 1.48 g of picric acid and 10 g of sodium hydroxide was mixed in 200cc iron container.
Purification
Making use of phenol’s high water solubility, and the higher solubility of dinitro and mononitrophenol, the picric acid can be purified to near analytical grade by recrystallization from a solvent mixture of 1 volume ethanol and 2 volumes water, roughly 9 mL of solvent being required per gram of picric acid [28].
The crystals were then removed by vacuum filtering, were vacuum dried in a dissector, and form into a nearly yellow mass of m.p 118°C. After that, calculate the yield of picric acid.
Equation:
X-OH +3HNO3 + H2SO4 → X-NO2
Where:
X-ON= Phenol
X-NO2= Picric acid
Yield:
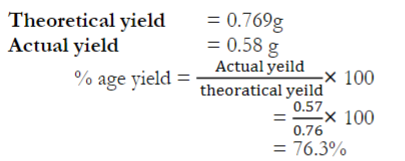
The amount of products obtain in a chemical reaction is called actual yield. %age yield can be calculated by dividing actual yield to theoretical yield and multiplying by 100.
Phenol : Picric Acid
94 g = 229 g
1 g = 229/94
0.941 g = 229/94×0.941 = 2.29 g
Synthesis of picramic acid
Preparation of solutions:
Preparation of 6.5 milimole of picric acid:
229 g of picric acid = 1000 milimole
1 g of picric acid = 1000/229 = 4.3668 milimole
1.48 g of picric acid = 4.3668x1.48 = 6.5 milimole
Preparation of 6.5 milimole of Na2S:
1000 milimole of Na2S = 78 g
1 milimole = 78/1000 = 0.078 g
6.5 milimole = 0.078x6.5 = 0.507 g
Preparation of 6.5 milimole of picramic acid:
1000 milimoles of picramic acid = 119 g
1 milimole = 119/1000
6.5 milimole = 119/1000x6.5
6.5 milimole = 0.773 g
Preparation of 6.5 milimole of NaOH:
1000 milimole of NaOH = 40 g
1 milimole = 40/1000
6.5 milimole = 40/1000x6.5 = 0.26 g
Preparation of 6.5 milimoles of Na2S2O3:
1000 milimole of Na2S2O3 = 158 g
1 milimole = 158/1000
6.5 milimole = 158/1000x6.5 = 1.027 g
Procedure
Take a glass or iron container. A solution of picric acid (1.48 g) and 35% NaOH (10 g) in 200cc. water was heated to 55°C then added a solution of crystalline sodium sulfide in 100cc. water with vigorous stirring over a period of 10 minutes. When this addition was completed, an additional pulverized picric acid (1 g) was added in teaspoon portions and simultaneously a solution of sodium sulfide (0.512 g) in 200cc. Water was introduced, the additions of the two reagents being completed at the same time (within about 10 minutes in all). If the temperature goes above 65°C, ice was added. Stirring was continued for 10 minutes more, and then the mixture was poured onto 200 grams of ice, precipitating the sodium picramate completely. After 10 hours, the mixture was filtered, and the precipitate was washed with 10 per cent salt solution. Free picramic acid was
obtained by dissolving the sodium salt in 200cc. water. Warming the solution to80°C and acidifying with dilute sulfuric acid, with constant stirring. The mixture which should be just acid to Congo red was allowed to cool and stand for 10 hours. The product was then filtered off, form into a nearly brown paste of m.p 167.50C.
Equation:
4 X−NO2 +6Na2S+7H2O → 4X−NH2 + 6NaOH + 3Na2S2O3
Where,
X-NO2=Picric acid
X-NH2=Picramic acid
3.4.4 Yield:
Picric acid : Picramic acid
229 g = 119 g
1 g = 119/229
1.48 g = 119/229×100
= 0.769 g
Synthesis of sodium picramate
Preparation of solutions:
Preparation of 5 milimoles of picramic acid:
1 mole of picramic acid = 199 g (1 mole=1000)
1000 milimole = 199 g
1 milimole = 199/1000
5 milimoles = 199/1000×5 = 0.99 g
Preparation of 5 milimoles of NaOH:
1 mole of NaOH = 40 g (1 mole=1000 milimoles)
1000 milimoles = 40 g
1 milimole = 40/1000
5 milimole =40/1000×5 = 0.2 g
Procedure
In a beaker added 50 mL warm water and sodium hydroxide (0.2 g). Mix those with a glass rod until all the NaOH has dissolved. Dissolve picramic acid (0.2 g) crystals in the NaOH-water solution by stirring. Allow the solution mixture to cool down. Filter this mixture through filter papers. Small red particles will gather on the paper [29,30,31,32]. Discard the liquid. Dissolve these red particles in 100 ml of boiling water. Remove and filter this hot liquid through a filter paper. Discard the particles left on the paper. On drying crystals of sodium picramate were formed with melting point 96.50C.
4 RESULTS AND DISCUSSION
Color of Compounds
The colors of compounds are given in table below:
Table 1: Color of compounds

Melting point
Melting point is the temperature at which the whole compound changes its state either it becomes liquid or decomposes. Melting points of compounds are in table given below:
Table 2: Melting points of compounds

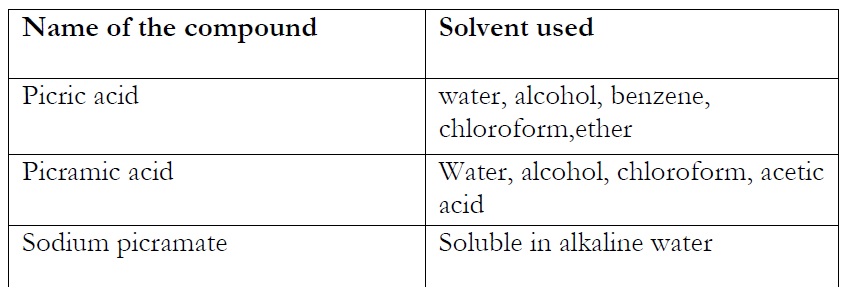
SOLUBILITY
Solubility of the compounds depends on the nature of compound. I checked the solubility of the compounds in different solvents. Their results are shown as follow:
Table3: Solubility of compounds
IR Data
The data of IR spectrum is summarized in the table given below:
Table 4: FTIR Spectrum (cm-1)
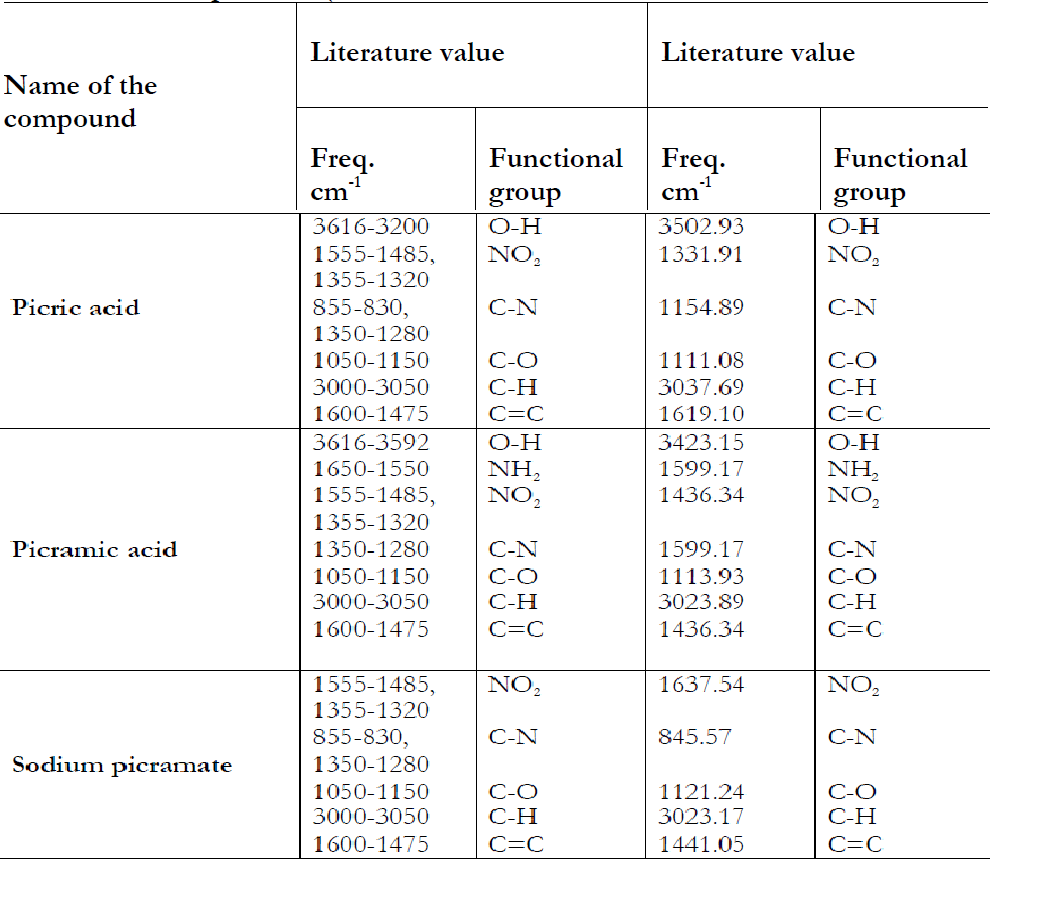
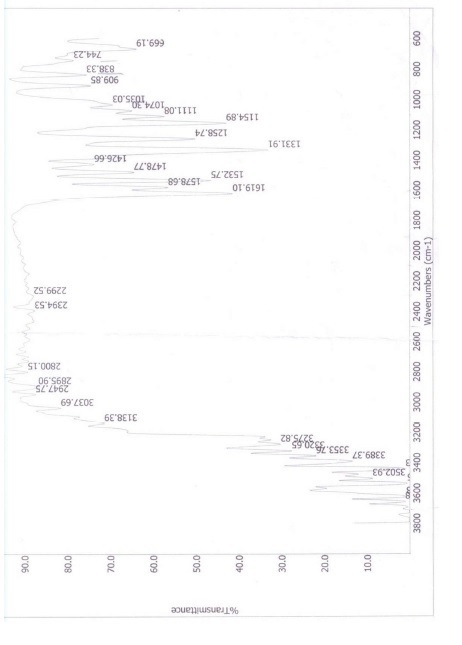
Figure5: IR Spectrum of picric acid

Figure6: IR Spectrum of picramic acid

Figure 7: IR spectrum of sodium picramate
Discussion
Three compounds Picric acid, Picramic acid and Sodium picramate were synthesized. Picramic acid and sodium picramate are the derivatives of Picric acid. The first compound Picric acid was formed by mixing Phenol, HNO3 and H2SO4 in the ratio 1:3:1. The second compound Picramic acid was formed by mixing Picric acid and Na2S in the ratio 1:2 followed by the addition of water. Similarly, the third compound was formed by mixing Picramic acid and NaOH in the ratio 1:1. The compounds were characterized by IR spectroscopy.
In first step synthesized compounds were confirmed by checking their physical properties i.e. color, solubility and further confirmation was done by their melting points in table 1, 2 and 3. In the first compound, the band at 3502.93 cm-1 was due to stretching vibration of OH Group. The NO2 frequency was observed at1331.91 cm-1. The C-N frequency was observed at 1154.89 cm-1. The C-O frequency was observed at 1111.08 cm-1. The CH
frequency was observed at 3037.69 cm-1. The C=C frequency was observed at 1619.10 cm-1.
In the second compound, the band at 3423.15 cm-1 was due to stretching vibration of O-H Group. The NH2 frequency was observed at 1599.17 cm-1. The NO2 frequency was observed at 1436.34 cm-1. The C-N frequency was observed at 1599.17 cm-1. The C-O frequency was observed at 1113.93 cm-1. The C-H frequency was observed at 3023.89 The C=C frequency was observed at 1436.34.
In the third compound the band at 1637.54 was due to NO2. The C-N frequency was observed at 845.57 cm-1. The C-O frequency was observed at 1121.24 cm-1. The C-H frequency was observed at 3023.17 cm-1. The C=C frequency was observed at 1441.05 cm-1.
Conclusions
Synthesis of Picric acid, Picramic acid and sodium Picramate were synthesized in excellent yield. All the compounds were characterized by FTIR. Both Picramic acid and Sodium picramate are the derivatives of Picric acid. It should be noted that Picric acid, Picramic acid and Sodium picramate could be easily formed through the nitration of phenol, reduction with sodium sulfide and treatment with sodium hydroxide respectively and have many industrial and biological applications. Picric acid used in medicinal formulations in the treatment of malaria, trichinosis, herpes, smallpox and antiseptics. It is used for dyeing wool, silk, and leather. It is frequently found in forensic laboratories for use in the Christmas tree stain and for Urine detection. Picramic acid and Sodium picramate are used to make dyes. Both Picramic Acid and Sodium Picramate are used as dyes in permanent hair dyes and colors.
Author’s Contribution. All the authors contributed equally.
Conflict of interest. We declare no conflict of interest for publishing this manuscript in IJIST.
Project details. NIL
REFFERENCES
1. Kadiyala, V. and Spain, J. C. A two-component monooxygenase catalyzes both the hydroxylation of p- nitrophenol and the oxidative release of nitrite from 4- nitrocatechol in Bacillus sphaericus, J.Appl. Environ. Microbiol. 64: 2479-2484 (1998).
2. Lenke, H. and Knack, H. J. M. Initial hydrogenation and extensive reduction of substituted 2, 4-dinitrophenols, J. Appl. Environ. Microbiol. 62: 784-790 (1996).
3. Furniss, B. S., Hannaford, A. J., Smith, P. W. G. and Tatchell, A. R. " Vogel’s Textbook of Practical Organic Chemistry," 4th ed. Longman, London and New York, (1986).
4. Gaensslen, R., Mertens, J., Lee, H. and Stolotow, M. “Staining and Extraction Techniques”, Proceeding of a Forensic Science Symposium on the Analysis of Sexual Assault Evidence, FBI Academy. (1983).
5. Becker, L. C., Bergfeld, W. F., Belsito, D. V., Klaassen, C. D., Marks, J. G.,Shank, R.C., Slaga, T. J., Snyder, P. W. And Andersen, A. F. Amended safety assessment of sodium picramate and picramic acid, J.Int. Toxicol. 6 (2): (2009).
6. Kadiyala, V. and Spain. J. C. A two-component monooxygenase catalyzes both the hydroxylation of p-nitrophenol and the oxidative release of nitrite from 4- nitrocatechol in Bacillus sphaericus, J. Appl. Environ. Microbial. 64: 2479-2484 (1998).
7. Oxford, G. S. and Pooler, J. P. Selective modification of sodium channel gating in lobster axons by 2, 4, 6-trinitrophenol: Evidence for two inactivation mechanisms, J. Gen. Physiol. 66 (6): 765-779 (1975).
8. Wyman, J. F., Guard, H. E., Won, W. D. and Quay, J. H. Conversion of 2, 4, 6- trinitrophenol to a mutagen by Pseudomonas aeruginosa, J. Appl. and Environ. Microbiol. 37 (2): 222-226 (1979).
9. Coombes, R. G. and Diggle, A. W. The Mechanism of nitration of phenol and 4- methylphenol by nitrogen dioxide in solution, 35, (34): 6373-6376, (1994).
10. Rieger, P. G. and Knackmuss, H. J. Basic Knowledge and Perspectives on Biodegradation of 2, 4, 6-Trinitrotoluene and Related Nitroaromatic Compounds in Contaminated Soil, Environ. Sci. Res. 49: 1-18 (1995).
11. Rieger, P. G., Sinnwell, V., Preu, A., Francke, W. And Knackmus, H. J. Hydride- Meisenheimer Complex Formation and Protonation as Key Reactions of 2, 4, 6- Trinitrophenol Biodegradation by Rhodococcuserythropolis, J. Becteriol. 181 (4): 1189-1195 (1999).
12. Behrend, C. and Wagner, K. H. Formation of Hydride-Meisenheimer Complexes of Picric acid (2, 4, 6-trinitrophenol) and 2, 4-dinitrophenol during Mineralization of picric acid by Nocardioides sp. Strain CB 22-2, J. Appl. and Environ. Microbiol. 65 (4): 1372-1377 (1999).
13. Ebert, S., Rieger, P. G. and Knackmuss, H. J. Function of Coenzyme F420 in Aerobic Catabolism of 2, 4, 6-Trinitrophenol and 2,4-Dinitrophenol by Nocardioides simplex FJ2-1A, J.Bicteriol. 181 (9): 2669-2674 (1999).
14. Gupta, V. K., Srivastava1, S. K. and Tyagi, R. Design parameters for the treatment of phenolic wastes by carbon columns (obtained from fertilizer waste material), 34 (5): 1543-1550 (2000).
15. Ahmed, S. A., Patil, R. C., Nakayama, M. and Ogura, K. Characterization of picric-acid-doped poly (o-toluidine) and the picric-acid-doped poly (o-toluidine)- induced conductive composite of acrylonitrile–butadiene–styrene, Synth. Met. 114 (2): 155-160 (2000).
16. Zolfigol, M. A., Ghaemi, E. and Madrakian, E. Nitration of Phenol under Mild and Heterogeneous Conditions, Mol.6: 614-620 (2001).
17. Dagade, S. P., Kadam, V. S. and Dongare, M. K. Regioselective nitration of phenol over solid acid catalysts, Catal. Commun.3: 67-70 (2002).
18. Ksibia, M., Zemzemia, A. and Boukchinab, R. Photocatalytic degradability of substituted phenols over UV irradiated TiO2, J. Photochem. And Photobiol. A: Chem. 159 (1): 61-70 (2003).
19. Noguchi, M., Onuki, T. and Mitamura, J. Hair dye composition. 2003. US. Pat. Appl. 10/148,408.
20. Shankaran, D. R., Gobi, K. V., Matsumoto, K., Imato, T., Toko, K. and Miura, N. Highly sensitive surface plasmon resonance immunosensor for parts-pertrillion level detection of 2, 4, 6-trinitrophenol, Sens. Actuators, B. Chem.100 (3): 450-454 (2004).
21. Nipper, M., Carr, R. S., Biedenbach, J. M., Hooten, D. L. and Miller, k. Fate and effects of picric acid and 2,6-DNT in marine environments: Toxicity of degradation products. Mar. Pollut. Bull. 55 (11): 1205-1217 (2005).
22. Srinivasan, P., Gunasekaran, M., Kanagasekaran, T., Gopalakrishnan, R. and Ramasamy, P. 2, 4, 6-trinitrophenol (TNP): An organic material for nonlinear optical (NLO) applications, J. Cryst. Growth. 289 (2):639-649 (2006).
23. Greenberg, N. A. and Shipe, W. F. Comparison of the abilities of tricbloroacetic, picric, sulfosalicylic, and tungstic acids to precipitate protein hydrolysate and proteins, J. Food Sci. 44(3): 735-737 (2006).
24. Weidhaas, J. L., Schroeder, E. D. and Chang, D. P. Y. An aerobic sequencing batch reactor for 2, 4,6 -trinitrophenol (picric acid) biodegradation, Biotechnol. And Bioeng. 97 (6): 1408-1414 (2007).
25. Goodfellow, W. L., Burton, D. T., Graves, W. C., Hall, L. W. and Cooper, K. R. Acute toxicity of picric acid and picramic acid to rainbow trout, salmogairdneri and American oyster, Crassostrea virginica, J. Am. water Resour. Assoc. 16 (4): 641- 648 (2007).
26. Shen, J., He, R., Yu, H., Wang, L., Zhang, J., Sun, X., Li, J., Han, W. and Xu, L. Biodegradation of 2, 4, 6-trinitrophenol (picric acid) in a biological aerated filter (BAF), Bioresour. Technol. 100 (6): 1922-1930 (2009).
27. Becker, L. C., Bergfeld, W. F., Belsito, D. V., Klaassen, C. D., Marks, J. G., Shank, R. C., Slaga, T. J., Synder, P. W. and Anderson, A. F. Amended safety assessment of sodium picramate and picramic acid, J. Int. Toxicol. 28(6): (2009).
28. Sheth, A., Doshi, N., Sen, D. J., Badmanaban, R. and Patel, C. N. Synthesis and biological screening of picric acid and amino phenols derivatives for antimicrobial activity, J. Chem. Pharm.2 (2): 1-12 (2010).
29. Khazaei, A., Zolfigol, M. A., Moosavi, A. R. and Zare, A. An efficient method for the nitration of phenols with NaNO acid imidazolium chloride, Chem. and Chem. Eng.
30. Pourali, A. R. and Goli, A. Nitration of phenolic compounds and oxidation of hydroquinone are using tetrabutylammonium chromate and dichromate under aprotic conditions, J. Chem. Sci.
31. Natarajan, S., Moovendaran, K., Sundar, J. K., Bhagavannarayana, G. and S.A. Martin, B. D. Influence of Picric Acid on the SHG Efficiency of L Tartrate Crystals, J. Miner. & Mater. Charact. & Eng.
32. Girija, P., Parthiban, S. and Mojumdar, S. C. Influence of picric acid on SHG efficiency of tris (thiourea) zinc(II) sulphate (ZTS), 119 (2): 953-958 (2015).
 |
Copyright © by authors and 50Sea. This work is licensed under Creative Commons Attribution 4.0 International License. |




















