Sequestration of Carbon Dioxide via Mineral Carbonation to Produce Magnesium Carbonate: A Design Study
Keywords:
Mineral Carbonation, Olivine, Magnesium Carbonate, Ex-Situ CarbonationAbstract
The rapid increase in atmospheric carbon dioxide (CO₂) due to industrialization and fossil fuel combustion has raised significant concerns about global warming. Carbon capture and storage (CCS) is a crucial technology for reducing greenhouse gas (GHG) emissions. This study presents the design of a mineral carbonation plant capable of sequestering 30 tons of CO₂ per day to produce magnesium carbonate (MgCO₃) using olivine as the feedstock.
The process follows an ex-situ carbonation approach, where a mineral slurry reacts with CO₂ under controlled conditions. The plant design includes the development of key equipment such as a reactor, heat exchanger, and flash column, with a detailed process flow diagram (PFD) modeled in Aspen Plus. Material and energy balances ensure the operational feasibility of the system.
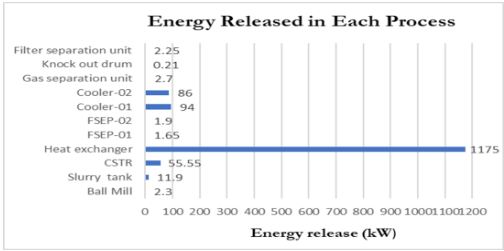
With an effective conversion rate of 50%, the process accounts for realistic industrial limitations while maintaining reliability at scale. Heat recovery mechanisms, including a shell-and-tube heat exchanger, improve energy efficiency by minimizing heat loss. Optimized equipment design ensures process scalability and aligns with performance criteria to meet sequestration targets and product quality standards.
The reliance on olivine, an abundant and cost-effective silicate mineral, highlights the economic and environmental advantages of this approach. The findings contribute to advancing sustainable CCS technologies, offering a viable solution for CO₂ mitigation while producing valuable industrial products such as MgCO₃ and by-product SiO₂.
References
A. K. Das and A. Sharma, “Climate change and the energy sector,” in Advancement in Oxygenated Fuels for Sustainable Development, Elsevier, 2023, pp. 1–6.
S. Pacala and R. Socolow, “Stabilization wedges: solving the climate problem for the next 50 years with current technologies,” Science (80-. )., vol. 305, no. 5686, pp. 968–972, 2004.
M. E. Boot-Handford et al., “Carbon capture and storage update,” Energy Environ. Sci., vol. 7, no. 1, pp. 130–189, 2014.
S. Ó. Snæbjörnsdóttir, B. Sigfússon, C. Marieni, D. Goldberg, S. R. Gislason, and E. H. Oelkers, “Carbon dioxide storage through mineral carbonation,” Nat. Rev. Earth Environ., vol. 1, no. 2, pp. 90–102, 2020.
R. M. Cuéllar Franca and A. Azapagic, “Life cycle environmental impacts of carbon capture, storage, and utilization,” Encycl. Sustain. Technol., pp. 447–459, 2017.
C. Cooper, “A technical basis for carbon dioxide storage,” Energy Procedia, vol. 1, no. 1, pp. 1727–1733, 2009.
B. Hitchon, W. D. Gunter, T. Gentzis, and R. T. Bailey, “Sedimentary basins and greenhouse gases: a serendipitous association,” Energy Convers. Manag., vol. 40, no. 8, pp. 825–843, 1999.
T. Gentzis, “Subsurface sequestration of carbon dioxide—an overview from an Alberta (Canada) perspective,” Int. J. Coal Geol., vol. 43, no. 1–4, pp. 287–305, 2000.
D. A. Voormeij and G. J. Simandl, “Geological, ocean, and mineral CO 2 sequestration options: a technical review,” Geosci. Canada, vol. 31, no. 1, pp. 11–22, 2004.
M. I. Rashid, Z. Yaqoob, M. A. Mujtaba, H. Fayaz, and C. A. Saleel, “Developments in mineral carbonation for Carbon sequestration,” Heliyon, 2023.
S. R. Ali and N. Mujahid, “Sectoral carbon dioxide emissions and environmental sustainability in Pakistan,” Environ. Sustain. Indic., vol. 23, p. 100448, 2024.
E. H. Oelkers, S. R. Gislason, and J. Matter, “Mineral carbonation of CO2,” Elements, vol. 4, no. 5, pp. 333–337, 2008.
A. A. Olajire, “A review of mineral carbonation technology in sequestration of CO2,” J. Pet. Sci. Eng., vol. 109, pp. 364–392, 2013, doi: 10.1016/j.petrol.2013.03.013.
R. Z. Juan Carlos Abanades, Rodney Allam, Klaus S. Lackner, Francis Meunier, Edward Rubin, Juan Carlos Sanchez, Katsunori Yogo, “Mineral carbonation and industrial uses of carbon dioxide,” Carbon Dioxide Capture and Storage, vol. 10, 2005.
Q. R. S. Miller, H. T. Schaef, J. P. Kaszuba, G. Gadikota, B. P. McGrail, and K. M. Rosso, “Quantitative review of olivine carbonation kinetics: reactivity trends, mechanistic insights, and research frontiers,” Environ. Sci. Technol. Lett., vol. 6, no. 8, pp. 431–442, 2019.
A. Saeed, “a Review of Mineral Carbonation By Enhanced,” vol. 31, no. 3, pp. 195–201, 2012.
and S. W. R. L. Cerro, B. G. Higgins, “Material balances for chemical engineers,” na, 2005.
R. C. Wouter Huijgen, Geert-Jan Witkamp, “Mineral CO2 sequestration in alkaline solid residues,” Greenh. Gas Control Technol. 7, vol. 2, no. 2, pp. 2415–2418, 2005, doi: https://doi.org/10.1016/B978-008044704-9/50344-X.
W. K. O’Connor, D. C. Dahlin, G. E. Rush, C. L. Dahlin, and W. K. Collins, “Carbon dioxide sequestration by direct mineral carbonation: process mineralogy of feed and products,” Mining, Metall. Explor., vol. 19, pp. 95–101, 2002.
R. M. Felder, R. W. Rousseau, and L. G. Bullard, Elementary principles of chemical processes. John Wiley & Sons, 2020.
W. L. McCabe, J. C. Smith, and P. Harriott, Unit operations of chemical engineering. McGraw-hill, 1993.
P. Trambouze, J.-P. Euzen, and R. Bononno, “Chemical reactors: from design to operation,” (No Title), 2004.
J. A. Williams, “COULSON AND RICHARDSON’S CHEMICAL ENGINEERING Volume 6 (Design), by RK Sinnott,” Chem. Eng. Educ., vol. 29, no. 2, p. 111, 1995.
I. Naderipour, “The Experimental Study on Effects of Height and Hold up on performance of Vertical Gas-Liquid Separator using Amin Contactor Tower,” Ciência e Nat., vol. 37, no. 6–1, pp. 93–103, 2015.
W. Svrcek and W. Monnery, “Design two-phase separators within,” Chem. Eng. Prog., 1993.
Downloads
Published
How to Cite
Issue
Section
License
Copyright (c) 2025 50sea

This work is licensed under a Creative Commons Attribution 4.0 International License.




















