Catalytic Performance of Electro-Oxidative Natural Manganese Sand for Ammonium Nitrogen Removal
Keywords:
Electrochemical Oxidation, Manganese Sand Catalyst, Ammonium Nitrogen Removal, Wastewater Treatment, SustainabilityAbstract
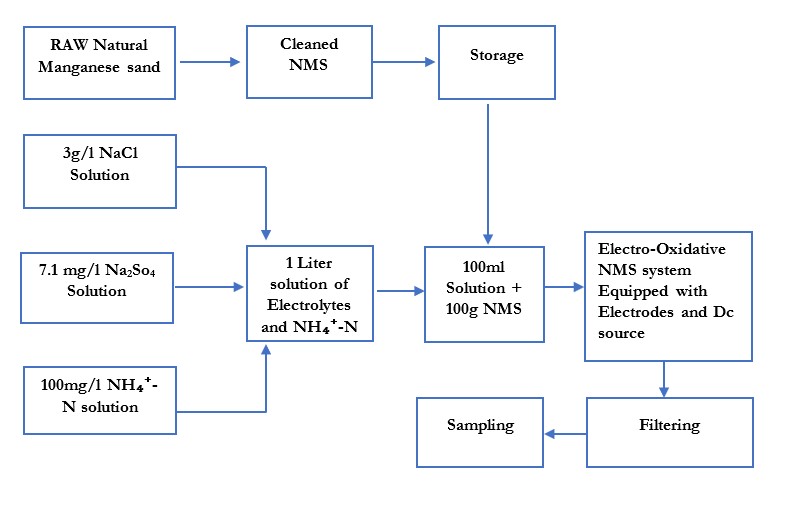
The environmental risks associated with ammonium nitrogen (NH₄⁺-N) pollution have led to a growing focus on prevention. Electrochemical advanced oxidation is an effective and eco-friendly method that only requires electricity and electrolytes to remove NH₄⁺-N from wastewater. This study assesses the effectiveness of electro-oxidative natural manganese sand (NMS) in removing ammonium nitrogen under different conditions. Due to NMS’s high redox potential, it significantly enhanced the electrochemical oxidation process, increasing NH₄⁺-N removal and generating reactive chlorine species (ClO⁻/HClO) when NaCl was added. The experiment was also conducted without a catalyst, quartz sand, and natural manganese sand, but NMS removed 86.4% of NH₄⁺-N, outperforming the other treatments. The removal efficiency was tested at five different pH levels (3, 5, 7, 9, and 11), with NMS showing the highest efficiency of 95.2% at pH 9. At a current density of 15.5 mA/cm², the removal rate reached 94.9%, and with a NaCl concentration of 9 g/L, the removal efficiency peaked at 96.2%, driven by increased production of reactive chlorine species (ClO⁻). These results demonstrate the electro-oxidative NMS system as a highly efficient, scalable, and eco-friendly solution for ammonium nitrogen removal in wastewater treatment.
References
T. Zhou, M. Wang, H. Zeng, R. Min, J. Wang, and G. Zhang, “Application of physicochemical techniques to the removal of ammonia nitrogen from water: a systematic review,” Environ. Geochemistry Heal. 2024 469, vol. 46, no. 9, pp. 1–24, Jul. 2024, doi: 10.1007/S10653-024-02129-6.
N. Sonadia, Z. Iqbal, W. Miran, A. Ul-Hamid, K. S. Ayub, and F. Azad, “Enhanced Electrocatalytic Performance of Erbium-Incorporated Nickel-Based Metal-Organic Frameworks for Water Splitting,” Energy and Fuels, vol. 38, no. 6, pp. 5397–5406, Mar. 2024, doi: 10.1021/ACS.ENERGYFUELS.3C04609/SUPPL_FILE/EF3C04609_SI_001.PDF.
V. R. Viktor Yushchenko, Еlena Velyugo, “Influence of ammonium nitrogen on the treatment efficiency of underground water at iron removal stations,” Groundw. Sustain. Dev., vol. 22, p. 100943, 2023, doi: https://doi.org/10.1016/j.gsd.2023.100943.
S. F. S. Lennevey Kinidi, Ivy Ai Wei Tan, Noraziah Binti Abdul Wahab, Khairul Fikri Bin Tamrin, Cirilo Nolasco Hipolito, “Recent Development in Ammonia Stripping Process for Industrial Wastewater Treatment,” Int. J. Chem. Eng., 2018, doi: https://doi.org/10.1155/2018/3181087.
R. S. Vinod K Gupta, H. Sadegh, Mehdi Yari, “Removal of ammonium ions from wastewater: A short review in development of efficient methods,” Global Journal of Environmental Science and Management. Accessed: Mar. 13, 2025. [Online]. Available: https://www.researchgate.net/publication/270341154_Removal_of_ammonium_ions_from_wastewater_A_short_review_in_development_of_efficient_methods
S. Xiang et al., “New progress of ammonia recovery during ammonia nitrogen removal from various wastewaters,” World J. Microbiol. Biotechnol. 2020 3610, vol. 36, no. 10, pp. 1–20, Aug. 2020, doi: 10.1007/S11274-020-02921-3.
S. W. Xiaolong Yang, Lihua Liu, “A strategy of high-efficient nitrogen removal by an ammonia-oxidizing bacterium consortium,” Bioresour. Technol., vol. 275, pp. 216–224, 2019, doi: https://doi.org/10.1016/j.biortech.2018.12.057.
Y. Cheng, T. Huang, Y. Sun, and X. Shi, “Catalytic oxidation removal of ammonium from groundwater by manganese oxides filter: Performance and mechanisms,” Chem. Eng. J., vol. 322, pp. 82–89, 2017, doi: https://doi.org/10.1016/j.cej.2017.04.010.
J. W. Xinyu Liu, “Selective oxidation of ammonium to nitrogen gas by advanced oxidation processes: Reactive species and oxidation mechanisms,” J. Environ. Chem. Eng., vol. 11, no. 3, p. 110263, 2023, doi: https://doi.org/10.1016/j.jece.2023.110263.
Z. Abbas et al., “Catalytic nonthermal plasma using efficient cobalt oxide catalyst for complete mineralization of toluene,” Res. Chem. Intermed., vol. 47, no. 6, pp. 2407–2420, Jun. 2021, doi: 10.1007/S11164-021-04406-W/METRICS.
L. Z. Fengjiao Quan, Guangming Zhan, Bing Zhou, Cancan Ling, Xiaobing Wang, Wenjuan Shen, Jianfen Li, Falong Jia, “Electrochemical removal of ammonium nitrogen in high efficiency and N2 selectivity using non-noble single-atomic iron catalyst,” J. Environ. Sci., vol. 125, pp. 544–552, 2023, doi: https://doi.org/10.1016/j.jes.2022.03.004.
W. W. Lixia Jia, Qi Zhou, Yuanwei Li, “Application of manganese oxides in wastewater treatment: Biogeochemical Mn cycling driven by bacteria,” Chemosphere, vol. 336, p. 139219, 2023, doi: https://doi.org/10.1016/j.chemosphere.2023.139219.
O. Scialdone, S. Randazzo, A. Galia, and G. Silvestri, “Electrochemical oxidation of organics in water: Role of operative parameters in the absence and in the presence of NaCl,” Water Res., vol. 43, no. 8, pp. 2260–2272, 2009, doi: https://doi.org/10.1016/j.watres.2009.02.014.
H. L. Kunning Lin, Yong Zhu, Yuanbiao Zhang, “Determination of ammonia nitrogen in natural waters: Recent advances and applications,” Trends Environ. Anal. Chem., vol. 24, p. e00073, 2019, doi: https://doi.org/10.1016/j.teac.2019.e00073.
C. Kim, T. T. Thao, J.-H. Kim, and I. Hwang, “Effects of the formation of reactive chlorine species on oxidation process using persulfate and nano zero-valent iron,” Chemosphere, vol. 150, p. 126266, 2020, doi: https://doi.org/10.1016/j.chemosphere.2020.126266.
Kwang-Wook Kim, Y.-J. Kim, I.-T. Kim, G.-I. Park, and E.-H. Lee, “The electrolytic decomposition mechanism of ammonia to nitrogen at an IrO2 anode,” Electrochim. Acta, vol. 50, no. 22, pp. 4356–4364, 2005, doi: https://doi.org/10.1016/j.electacta.2005.01.046.
X. T. Qian Peng, Yingjie Zhang, Wanling Zhong, Kun Liu, Jiajie Xing, “Facile preparation of manganese sand-based monolithic catalysts with excellent catalytic performance and reusability for activation of peroxymonosulfate: The key role of pre-calcination,” J. Water Process Eng., vol. 56, p. 104398, 2023, doi: https://doi.org/10.1016/j.jwpe.2023.104398.
D. . Mehendale, F. V, Clayton, G., Homyer, K., Reynolds, “HOCl vs OCl−: clarification on chlorine-based disinfectants used within clinical settings,” J. Glob. Heal. Reports, vol. 7, 2023, doi: https://doi.org/10.29392/001c.84488.
S.-E. O. Umesh Ghimire, Min Jang, Sokhee P. Jung, Daeryong Park, Se Jin Park, Hanchao Yu, “Electrochemical Removal of Ammonium Nitrogen and COD of Domestic Wastewater using Platinum Coated Titanium as an Anode Electrode,” Energies, vol. 12, no. 5, p. 883, 2019, doi: https://doi.org/10.3390/en12050883.
T. D. W. Changyong Zhang, Di He, Jinxing Ma, “Active chlorine mediated ammonia oxidation revisited: Reaction mechanism, kinetic modelling and implications,” Water Res., vol. 145, pp. 220–230, 2018, doi: https://doi.org/10.1016/j.watres.2018.08.025.
J. Shu, R. Liu, Z. Liu, H. Chen, and C. Tao, “Leaching of manganese from electrolytic manganese residue by electro-reduction,” Environ. Technol., vol. 38, no. 16, pp. 2077–2084, Aug. 2017, doi: 10.1080/09593330.2016.1245789.
Downloads
Published
How to Cite
Issue
Section
License
Copyright (c) 2025 50Sea

This work is licensed under a Creative Commons Attribution 4.0 International License.




















