Synergistic Effect of Pyrolyzed Bagasse and Trichoderma Viride for Sustainable Mitigation of Chili Southern Blight
Keywords:
Organic Amendments, Plant Protection, Sclerotium Rolfsen, Biological Control, XRDAbstract
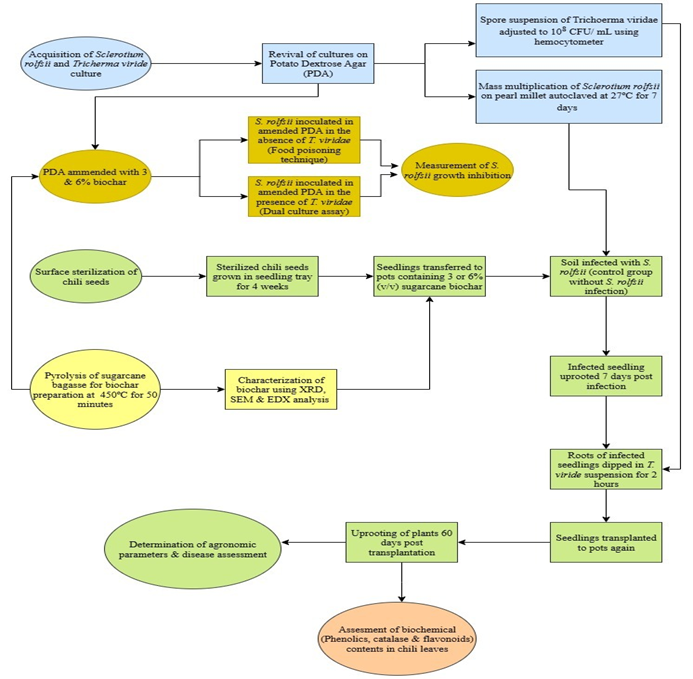
Soil-borne diseases like southern blight severely limit chili (Capsicum annuum L.) production, demanding sustainable and eco-friendly management approaches. This study introduces the integration of sugarcane bagasse-derived biochar with Trichoderma viride as a novel strategy for enhancing chili resistance against Sclerotium rolfsii. Biochar was produced through pyrolysis at 450°C and characterized using SEM, EDX, and XRD, revealing porous honeycomb-like structures, high carbon content, and mineral phases such as SiO₂ and CaO. Glasshouse experiments were conducted on the chili cultivar ‘Desi’ using biochar at 3% & 6% (v/v) concentrations. Biochar was either applied alone or in combination with T. viride as well as with S. rolfsii. Results demonstrated that biochar treatments significantly enhanced shoot and root growth, biomass accumulation, and physiological performance under pathogen stress. Disease severity, incidence, and mortality were notably reduced, with the greatest suppression (20%) noted in chili plants treated with 6% biochar plus T. viride. Furthermore, higher biochar doses substantially elevated levels of defense-related compounds, including phenolics, catalase, and flavonoids, indicating induction of systemic resistance. Similarly, the combined effect of biochar and T. viride was also visible under in vitro assays. Overall, the integration of biochar and beneficial fungi not only improved soil health but also strengthened host defense, offering a sustainable approach to managing southern blight. These findings highlight biochar-induced resistance as a promising component of integrated disease management in chili cultivation.
References
F. R. Amin, Y. Huang, Y. He, R. Zhang, G. Liu, and C. Chen, “Biochar applications and modern techniques for characterization,” Clean Technol. Environ. Policy, vol. 18, no. 5, pp. 1457–1473, Jun. 2016, doi: 10.1007/S10098-016-1218-8/METRICS.
G. of P. (GoP)., “Agricultural Statistics of Pakistan 2021–22,” Minist. Natl. Food Secur. Res. Islam. Pakistan., 2022, [Online]. Available: https://mnfsr.gov.pk/PublicationDetail/NmRlYjkzNDMtZGY1NS00Y2RjLTljZmItNmY0ODM4MmVhYWY0
S. Ahmad et al., “Effect of Application of Biochar, Poultry and Farmyard Manures in Combination with Synthetic Fertilizers on Soil Fertility and Cotton Productivity under Arid Environment,” Commun. Soil Sci. Plant Anal., vol. 52, no. 17, pp. 2018–2031, Sep. 2021, doi: 10.1080/00103624.2021.1908324.
N. Meena, K. C., Patel, J., Naruka, I. S., Haldar, A., Patidar, D. K., & Soni, “A Study of Growth Rate and Seed Yield in Ocimum basilicum Germplasms,” Int. J. Econ. Plants, vol. 9, pp. 111–114, 2022, [Online]. Available: https://ojs.pphouse.org/index.php/IJEP/article/view/4722
P. Paparu et al., “Morphological and Pathogenic Characterization of Sclerotium rolfsii, the Causal Agent of Southern Blight Disease on Common Bean in Uganda,” https://doi.org/10.1094/PDIS-10-19-2144-RE, vol. 104, no. 8, pp. 2130–2137, Jun. 2020, doi: 10.1094/PDIS-10-19-2144-RE.
M. A. Sulaiman and S. K. Bello, “Biological control of soil-borne pathogens in arid lands: a review,” J. Plant Dis. Prot., vol. 131, no. 2, pp. 293–313, Apr. 2024, doi: 10.1007/S41348-023-00824-7/METRICS.
A. Begum, S. N. Alam, and M. J. Uddin, “Management of Pesticides: Purposes, Uses, and Concerns,” Pestic. Residue Foods Sources, Manag. Control, pp. 53–86, Apr. 2017, doi: 10.1007/978-3-319-52683-6_4.
S. Ruqiya et al., “Biocontrol potential and molecular characterization of lipopeptides producing Bacillus subtilis against Sclerotinia sclerotiorum,” J. Biol. Control, vol. 36, no. 4, pp. 215–221, Dec. 2022, doi: 10.18311/JBC/2022/33785.
A. Muhibbudin, E. Mulyaningtyas Setiyowati, A. Wahyu Sektiono Jurusan Hama dan Penyakit Tumbuhan, F. Pertanian, U. Brawijaya Jl Veteran, and J. Timur, “MECHANISM ANTAGONISM of Trichoderma viride AGAINST SEVERAL TYPES of PATHOGENS and PRODUCTION of SECONDARY METABOLITES,” AGROSAINTIFIKA, vol. 4, no. 1, pp. 243–253, Jan. 2021, doi: 10.32764/AGROSAINTIFIKA.V4I1.2375.
A. Javaid, R. Afzal, and A. Shoaib, “Biological management of southern blight of chili by Penicillium oxalicum and leaves of Eucalyptus citriodora,” Int. J. Agric. Biol., vol. 23, no. 1, pp. 93–102, 2020, doi: 10.17957/IJAB/15.1263.
E. T. Abdou, A. M.A. Hammad, and A. M. Gerish, “Efficacy of Bioagent in Controlling Soil Borne Fungi and Assessment of Seed Yield and its Components in Peanut (Arachis Hypogea),” Alexandria Sci. Exch. J., vol. 45, no. 2, pp. 331–342, Jun. 2024, doi: 10.21608/ASEJAIQJSAE.2024.367638.
J. Poveda, Á. Martínez-Gómez, C. Fenoll, and C. Escobar, “The Use of Biochar for Plant Pathogen Control,” https://doi.org/10.1094/PHYTO-06-20-0248-RVW, vol. 111, no. 9, pp. 1490–1499, Oct. 2021, doi: 10.1094/PHYTO-06-20-0248-RVW.
M. Kolton, E. R. Graber, L. Tsehansky, Y. Elad, and E. Cytryn, “Biochar-stimulated plant performance is strongly linked to microbial diversity and metabolic potential in the rhizosphere,” New Phytol., vol. 213, no. 3, pp. 1393–1404, Feb. 2017, doi: 10.1111/NPH.14253.
R. Singh, A. Goyal, and S. Sinha, “Global insights into biochar: Production, sustainable applications, and market dynamics,” Biomass and Bioenergy, vol. 194, p. 107663, Mar. 2025, doi: 10.1016/J.BIOMBIOE.2025.107663.
J. Lin et al., “Effect of degradable microplastics, biochar and their coexistence on soil organic matter decomposition: A critical review,” TrAC Trends Anal. Chem., vol. 183, p. 118082, Feb. 2025, doi: 10.1016/J.TRAC.2024.118082.
C. Singh, P. Pathak, N. Chaudhary, and D. Vyas, “Production of biochar using top-lit updraft and its application in horticulture,” Sustain. Agric. Tech. Progress. Transitions, pp. 159–172, Oct. 2021, doi: 10.1007/978-3-030-83066-3_9.
X. Ma et al., “Study of Biochar Properties by Scanning Electron Microscope – Energy Dispersive X-Ray Spectroscopy (SEM-EDX),” Commun. Soil Sci. Plant Anal., vol. 47, no. 5, pp. 593–601, Mar. 2016, doi: 10.1080/00103624.2016.1146742.
“Management of southern blight of bell pepper by soil amendment with dry biomass of Datura metel on JSTOR.” Accessed: Sep. 18, 2025. [Online]. Available: https://www.jstor.org/stable/48763973
F. A. Mohiddin et al., “Development of Trichoderma based bio-formulations for the management of chilli wilt,” J. Pharmacogn. Phytochem., vol. 7, no. 1, pp. 2118–2122, Feb. 2018, Accessed: Sep. 18, 2025. [Online]. Available: https://www.phytojournal.com/archives/2018.v7.i1.2873/development-of-trichoderma-based-bio-formulations-for-the-management-of-chilli-wilt
F. Ahmad, K. Kusumiyati, M. A. Soleh, M. R. Khan, and R. S. Sundari, “Chili crop innovation: Exploring enclosed growing designs for varied varieties—A review,” Agrosystems, Geosci. Environ., vol. 7, no. 2, p. e20491, Jun. 2024, doi: 10.1002/AGG2.20491.
F. Ahmad, K. Kusumiyati, M. A. Soleh, M. R. Khan, and R. S. Sundari, “Chili cultivars vulnerability: a multi-factorial examination of disease and pest-induced yield decline across different growing microclimates and watering regimens,” BMC Plant Biol., vol. 24, no. 1, pp. 1–17, Dec. 2024, doi: 10.1186/S12870-024-05541-3/TABLES/8.
G. Riaz et al., “Formulation of the encapsulated rhizospheric Ochrobactrum ciceri supplemented with alginate for potential antifungal activity against the chili collar rot pathogen,” South African J. Bot., vol. 161, pp. 586–598, Oct. 2023, doi: 10.1016/J.SAJB.2023.08.048.
J. Bijali, T. Halder, and K. Acharya, “Elucidation of the biochemical and molecular basis of the differential disease expression in two cultivars of chili (Capsicum annuum) in response to Colletotrichum capsici infection,” Acta Physiol. Plant., vol. 43, no. 12, pp. 1–16, Dec. 2021, doi: 10.1007/S11738-021-03334-X/METRICS.
L. Corell, S. Armenta, F. A. Esteve-Turrillas, and M. de la Guardia, “Flavonoid determination in onion, chili and leek by hard cap espresso extraction and liquid chromatography with diode array detection,” Microchem. J., vol. 140, pp. 74–79, Jul. 2018, doi: 10.1016/J.MICROC.2018.04.014.
M. M. K. Shereen A. Soliman, “Evaluation of the Antifungal Activity of Bacillus amyloliquefaciens and B. velezensis and Characterization of the Bioactive Secondary Metabolites Produced against Plant Pathogenic Fungi,” Biology (Basel)., vol. 11, no. 10, p. 1390, 2022, doi: https://doi.org/10.3390/biology11101390.
M. Rasool, A. Akhter, G. Soja, and M. S. Haider, “Role of biochar, compost and plant growth promoting rhizobacteria in the management of tomato early blight disease,” Sci. Rep., vol. 11, no. 1, pp. 1–16, Dec. 2021, doi: 10.1038/S41598-021-85633-4;SUBJMETA.
L. Leng et al., “An overview on engineering the surface area and porosity of biochar,” Sci. Total Environ., vol. 763, p. 144204, Apr. 2021, doi: 10.1016/J.SCITOTENV.2020.144204.
M. F. Nieto-Jacobo et al., “Environmental growth conditions of trichoderma spp. Affects indole acetic acid derivatives, volatile organic compounds, and plant growth promotion,” Front. Plant Sci., vol. 8, p. 245069, Feb. 2017, doi: 10.3389/FPLS.2017.00102/BIBTEX.
O. Frenkel, A. K. Jaiswal, Y. Elad, B. Lew, C. Kammann, and E. R. Graber, “The effect of biochar on plant diseases: what should we learn while designing biochar substrates?,” J. Environ. Eng. Landsc. Manag., vol. 25, no. 2, pp. 105–113, Apr. 2017, doi: 10.3846/16486897.2017.1307202.
J. Nepal, W. Ahmad, F. Munsif, A. Khan, and Z. Zou, “Advances and prospects of biochar in improving soil fertility, biochemical quality, and environmental applications,” Front. Environ. Sci., vol. 11, p. 1114752, Feb. 2023, doi: 10.3389/FENVS.2023.1114752/FULL.
H. A. Contreras-Cornejo, L. Macías-Rodríguez, C. Cortés-Penagos, and J. López-Bucio, “Trichoderma virens, a Plant Beneficial Fungus, Enhances Biomass Production and Promotes Lateral Root Growth through an Auxin-Dependent Mechanism in Arabidopsis,” Plant Physiol., vol. 149, no. 3, pp. 1579–1592, Mar. 2009, doi: 10.1104/PP.108.130369.
M. Keiluweit, P. S. Nico, M. Johnson, and M. Kleber, “Dynamic Molecular Structure of Plant Biomass-Derived Black Carbon (Biochar),” Environ. Sci. Technol., vol. 44, no. 4, pp. 1247–1253, 2010, doi: 10.1021/ES9031419.
“(PDF) Characterization and evaluation of biochars derived from agricultural waste biomass from Gansu, China.” Accessed: Sep. 18, 2025. [Online]. Available: https://www.researchgate.net/publication/320371719_Characterization_and_evaluation_of_biochars_derived_from_agricultural_waste_biomass_from_Gansu_China
K. Y. Chan, L. Van Zwieten, I. Meszaros, A. Downie, and S. Joseph, “Agronomic values of greenwaste biochar as a soil amendment,” Soil Res., vol. 45, no. 8, pp. 629–634, Dec. 2007, doi: 10.1071/SR07109.
G. Wang, Y. Ma, H. Y. Chenia, R. Govinden, J. Luo, and G. Ren, “Biochar-Mediated Control of Phytophthora Blight of Pepper Is Closely Related to the Improvement of the Rhizosphere Fungal Community,” Front. Microbiol., vol. 11, p. 538973, Jul. 2020, doi: 10.3389/FMICB.2020.01427/BIBTEX.
G. E. Harman, C. R. Howell, A. Viterbo, I. Chet, and M. Lorito, “Trichoderma species — opportunistic, avirulent plant symbionts,” Nat. Rev. Microbiol. 2004 21, vol. 2, no. 1, pp. 43–56, Jan. 2004, doi: 10.1038/nrmicro797.
A. Mhamdi, G. Queval, S. Chaouch, S. Vanderauwera, F. Van Breusegem, and G. Noctor, “Catalase function in plants: a focus on Arabidopsis mutants as stress-mimic models,” J. Exp. Bot., vol. 61, no. 15, pp. 4197–4220, Oct. 2010, doi: 10.1093/JXB/ERQ282.
M. Villavicencio-Vásquez, F. Espinoza-Lozano, L. Espinoza-Lozano, and J. Coronel-León, “Biological control agents: mechanisms of action, selection, formulation and challenges in agriculture,” Front. Agron., vol. 7, p. 1578915, May 2025, doi: 10.3389/FAGRO.2025.1578915/FULL.
Downloads
Published
How to Cite
Issue
Section
License
Copyright (c) 2025 50sea

This work is licensed under a Creative Commons Attribution 4.0 International License.




















