Arsenic (v) Adsorption by Using Synthesized Iron Oxide Nanoparticles (Fe2O3-NPs) and Aluminum Oxide Nanoparticles (Al2O3-NPs)
Keywords:
Arsenic, Iron Oxide, Aluminum Oxide, Adsorption of Arsenic, AdsorbentsAbstract
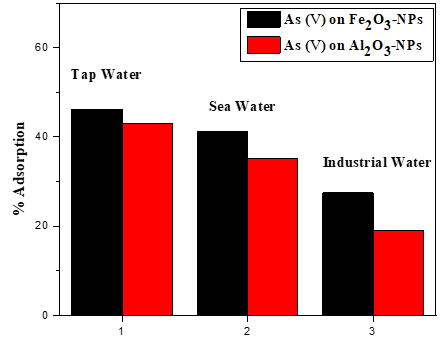
Arsenic, is one of the most harmful elements to human health that continuously causes a threat to the world. Arsenic is found in combined form in rocks under the earth's surface and when it dissolves, it contaminates groundwater. The current research synthesized iron oxide nanoparticles (Fe2O3-NPs) and aluminum oxide nanoparticles (Al2O3-NPs) for removal of arsenic (As) (˅) from an aqueous medium and characterized the synthesized material by different analytical techniques such as FT-IR spectroscopy and XRD spectroscopy. The results show successful synthesis of Fe2O3-NPs and Al2O3-NPs. Furthermore, the synthesized material was used as an adsorbent for extraction of as (V) from water. The effect of different parameters such as pH, temperature, contact time, and adsorbent dose on the adsorption process was investigated. The adsorption efficiency was determined by Fe2O3-NPs at about 20 mg/g and Al2O3-NPs at 19.5 mg/g. The quantitative removal of as (V) from industrial water required a minimum amount (0.2 g) of Fe2O3-NPs and Al2O3-NPs. various kinetic and isotherms were investigated in the current study. The result showed that the obtained data for Fe2O3-NPs was more fitted to Pseudo second order kinetic and Freundlich equation, while for Al2O3-NPs the data was more fitted to Pseudo second order kinetic and Elovich model equation, which confirms the interaction among as (V) and adsorbents. Thermodynamic parameters were also investigated which shows the process is spontaneous and endothermic. This model was used to estimate the site energy distribution for each adsorbent. Thermodynamic parameters were also investigated which shows the non-spontaneous and endothermic nature of the adsorbent. According to the results of the analysis of the approximate site energy distribution, adding Fe2O3 and Al2O3-NPs to arsenic decreased the area under the frequency distribution curve of the sorption site energies, which in turn decreased the number of sorption sites that were open to arsenic. This might be explained by the hydrophobic interaction between synthesized materials and arsenic being reduced due to the blocking of the Fe2O3 and Al2O3-NPs hydrophobic surface.
References
H. F. Bakhat, S. Arshad, S. Abbas, G. Shah, S. Fahad, H.M. Hammad & M. Shahid, (2022). Genotypic differences among the rice genotypes to arsenic stress cultivated under two water regimes: with an inference to human health. Journal of Plant Growth Regulation, 41(2), 558-568.
I. Carabante, (2012), Arsenic (V) adsorption on iron oxide: implications for soil remedeation and water purification.
A. Figoli, I. Fuoco, C. Apollaro, M. Chabane, R. Mancuso, B. Gabriele, & A. Criscuoli, (2020), Arsenic-contaminated groundwaters remediation by nanofiltration, Separation and Purification Technology, 238, 116461.
M. Vaclavikova, G. P. Gallios, S. Hredzak, & S. Jakabsky, (2008), Removal of arsenic from water streams: an overview of available techniques, Clean Technologies and Environmental Policy, 10(1), 89-95.
S. I. Siddiqui and S. A. Chaudhry, (2017), Iron oxide and its modified forms as an adsorbent for arsenic removal: a comprehensive recent advancement, Process Safety and Environmental Protection, 111, 592-626.
F. Akhlaghian, B. Souri and Z. Mohamadi, (2017), Nanostructured Fe2O3/Al2O3 adsorbent for removal of As (V) from water, Advances in Environmental Technology, 3(2), 67-75.
J. Jeong, M. Fan, S. Singh, C. Chuang and J. H. Van Leeuwen, (2007), Evaluation of iron oxide and aluminum oxide as potential arsenic (V) adsorbents, Chemical Engineering and Processing-Process Intensification, 46(10), 1030-1039.
S. Kundu, A.K. Gupta, Analysis, (2005), Analysis and modeling of fixed bed column operations on As (V) removal by adsorption onto iron oxide-coated cement (IOCC), Journal of colloid and interface Science, 290, 52–60.
S. Kundu, and A. K. Gupta, (2006), Arsenic adsorption onto iron oxide-coated cement (IOCC): regression analysis of equilibrium data with several isotherm models and their optimization. Chemical Engineering Journal, 122(1-2), 93-106.
L. Hao, M. Liu, N. Wang and G. Li, (2018), A critical review on arsenic removal from water using iron-based adsorbents. RSC advances, 8(69), 39545-39560.
H. Cui, Q. Li, S. Gao, and J. K. Shang, (2012), Strong adsorption of arsenic species by amorphous zirconium oxide nanoparticles, Journal of Industrial and Engineering Chemistry, 18(4), 1418-1427.
L. Yan, H. Tu, T. Chan and C. Jing, (2017), Mechanistic study of simultaneous arsenic and fluoride removal using granular TiO2-La adsorbent, Chemical Engineering Journal, 313, 983-992.
D. Liu, S. Deng, A. Maimaiti, B. Wang, J. Huang, Y. Wang and G. Yu, (2018), As (III) and As (V) adsorption on nanocomposite of hydrated zirconium oxide coated carbon nanotubes. Journal of colloid and interface science, 511, 277-284.
H. Gomaa, M. A. Shenashen, H. Yamaguchi, A. S. Alamoudi, M. Abdelmottaleb, M. F. Cheira and A. S. El-Safty, (2018), Highly-efficient removal of As (V), Pb2+, Fe3+, and Al3+ pollutants from water using hierarchical, microscopic TiO2 and TiOF2 adsorbents through batch and fixed-bed columnar techniques. Journal of cleaner production, 182, 910-925.
B. F. Urbano, I. Villenas, B. L. Rivas and C. H. Campos, (2015), Cationic polymer–TiO2 nanocomposite sorbent for arsenate removal, Chemical Engineering Journal, 268, 362-370.
P. Benjwal, M. Kumar, P. Chamoli and K. K. Kar, (2015), Enhanced photocatalytic degradation of methylene blue and adsorption of arsenic (iii) by reduced graphene oxide (rGO)–metal oxide (TiO2/Fe3O 4) based nanocomposites, Rsc Advances, 5(89), 73249-73260.
H. Chen, J. Li, X. Wu and X. Wang, (2014), Synthesis of alumina-modified cigarette soot carbon as an adsorbent for efficient arsenate removal. Industrial & Engineering Chemistry Research, 53(41), 16051-16060.
W. Li, D. Chen, F. Xia, J. Z. Tan, P. P. Huang, W. G. Song and R. A. Caruso, (2016), Extremely high arsenic removal capacity for mesoporous aluminium magnesium oxide composites. Environmental Science: Nano, 3(1), 94-106.
P.K. Mishra, P. Gahlyan, R. Kumar, P.K. Rai, ACS Sustainable Chem. Eng. 6 (2018) 10668–10678.
T. S. Sakthivel, S. Das, C. J. Pratt and S. Seal, (2017), One-pot synthesis of a ceria–graphene oxide composite for the efficient removal of arsenic species, Nanoscale, 9(10), 3367-3374.
B. Chen, Z. Zhu, J. Hong, Z. Wen, J. Ma, Y. Qiu and J. Chen, (2014), Nanocasted synthesis of ordered mesoporous cerium iron mixed oxide and its excellent performances for As (V) and Cr (VI) removal from aqueous solutions. Dalton Transactions, 43(28), 10767-10777.
J. Chen, J. Wang, G. Zhang, Q. Wu and D. Wang, (2018), Facile fabrication of nanostructured cerium-manganese binary oxide for enhanced arsenite removal from water, Chemical Engineering Journal, 334, 1518-1526.
L. Zhang, T. Zhu, X. Liu and W. Zhang, (2016), Simultaneous oxidation and adsorption of As (III) from water by cerium modified chitosan ultrafine nanobiosorbent. Journal of hazardous materials, 308, 1-10.
Z. Wen, J. Lu, Y. Zhang, G. Cheng, S. Huang, J. Chen and R. Chen, (2020), Facile inverse micelle fabrication of magnetic ordered mesoporous iron cerium bimetal oxides with excellent performance for arsenic removal from water, Journal of Hazardous Materials, 383, 121172.
L. Zhang, T. Zhu, X. Liu and W. Zhang, (2016), Simultaneous oxidation and adsorption of As (III) from water by cerium modified chitosan ultrafine nanobiosorbent, Journal of hazardous materials, 308, 1-10.
N. Inchaurrondo, C. Di Luca, F. Mori, A. Pintar, G. Žerjav, M. Valiente, and C. Palet, (2019), Synthesis and adsorption behavior of mesoporous alumina and Fe-doped alumina for the removal of dominant arsenic species in contaminated waters, Journal of environmental chemical engineering, 7(1), 102901.
D. Ociński, I. Jacukowicz-Sobala, P. Mazur, J. Raczyk and E. Kociołek-Balawejder, (2016), Water treatment residuals containing iron and manganese oxides for arsenic removal from water–Characterization of physicochemical properties and adsorption studies, Chemical Engineering Journal, 294, 210-221.
W. Zhang, C. Liu, T. Zheng, J. Ma, G. Zhang, G. Ren and Y. Liu, (2018), Efficient oxidation and sorption of arsenite using a novel titanium (IV)-manganese (IV) binary oxide sorbent. Journal of hazardous materials, 353, 410-420.
S. Guo, W. Sun, W. Yang, Q. Li, and J. K. Shang, (2015), Superior As (III) removal performance of hydrous MnOOH nanorods from water, RSC Advances, 5(66), 53280-53288.
S. Kumar, R. R. Nair, P. B. Pillai, S. N. Gupta, M. A. R. Iyengar and A. K. Sood, (2014), Graphene oxide–MnFe2O4 magnetic nanohybrids for efficient removal of lead and arsenic from water, ACS applied materials & interfaces, 6(20), 17426-17436.
Q. Zhang, J. Teng, G. Zou, Q. Peng, Q. Du, T. Jiao and J. Xiang, (2016), efficient phosphate sequestration for water purification by unique sandwich-like MXene/magnetic iron oxide nanocomposites. Nanoscale, 8(13), 7085-7093.
F. Xiao, L. Fang, W. Li, and D. Wang, (2015), One-step synthesis of aluminum magnesium oxide nanocomposites for simultaneous removal of arsenic and lead ions in water. RSC advances, 5(11), 8190-8193.
EA. Deliyanni, DN. Bakoyannakis, AI. Zouboulis, KA. Matis, (2003), Sorption of As(V) ions by akagane´ite-type nanocrystals, Chemosphere 50:155–163.
J. Youngran, F. A. Maohong and J. F. Belczyk, (2007), Effect of competing solutes on arsenic (V) adsorption using iron and aluminum oxides. Journal of Environmental Sciences, 19(8), 910-919.
KA. Matis, M. Lehmann, AI. Zouboulis, (1999), Modelling sorption of metals from aqueous solution onto mineral particles: the case of arsenic ions and goethite ore, In: Misaelides et al. (Eds) Natural microporous materials in environmental technology. Kluwer, The Netherlands, pp 463–472
JA. Wilkie, JG. Hering, (1996), Adsorption of arsenic onto hydrous ferric oxide: effect on adsorbate/adsorbent ratios and co-occurring solutes, Colloids Surf A Physicochem Eng Aspects, 107:97– 110.
OS. Thirunavukkaresu, T. Viraraghavan, KS. Subramanian, (2003), Arsenic removal from drinking water using iron oxide-coated sand, Water Air Soil Pollut 142:95–111.
I. Rau, A. Gonzalo, M. Valiente, (2003), Arsenic (V) adsorption by imobilized iron mediation, Modeling of the adsorption process and influence of interfering anions. React Funct Polym, 54:85–94.
Y. Zhang, M. Yang, X. Huang, (2003), Arsenic(V) removal with a Ce (IV)-doped iron oxide adsorbent, Chemosphere, 51:945–952.
W. Zhang, P. Singh, E. Paling, S. Delides, (2004), Arsenic removal from contaminated water by natural iron ores, Miner Eng, 17:517–524.
M. Vaclavikova, M. Matik, G. Gallios, S. Jakabsky, S. Hredzak, (2005a), The Synthesis and characterization of Fe nanostructures inside porous zeolites and their applications in water treatment technologies. In: Popov V, Lambin P (eds) Carbon nanotubes. Springer, UK pp, 239–240.
J. Hlavay, K. Polyak, (2005), Determination of surface properties of ironhydrocide-coated alumina adsorbent prepared for removal of arsenic from drinking water, J Colloid Interf Sci, 284:71–77.
HS. Altundogan, S. Altundogan, F. Tumen, M. Bildik, (2002), Arsenic adsorption from aqueous solutions by activated red mud, Waste Manage, 22:357–363.
AF. Bertocchi, M. Ghiani, R. Peretti, A. Zucca, (2006), Red Mud and fly ash for the remediation of mine sites contaminated with As, Cd, Cu, Pb and Zn, J Hazard Mater B 134:112–119
Lassoued, A., Dkhil, B., Gadri, A., & Ammar, S. (2017). Control of the shape and size of iron oxide (α-Fe2O3) nanoparticles synthesized through the chemical precipitation method. Results in physics, 7, 3007-3015.
Su, X., Chen, S., & Zhou, Z. (2012). Synthesis and characterization of monodisperse porous α-Al2O3 nanoparticles. Applied surface science, 258(15), 5712-5715.
F. Wang, X. Qin, Y. Meng, Z. Guo, L. Yang, and Y. Ming, “Hydrothermal synthesis and characterization of α-Fe2O3 nanoparticles,” Materials Science in Semiconductor Processing, vol. 16, no. 3, pp. 802–806, 2013.
P. Mallick and B. Dash, “Chemical Capping Synthesis of Nickel Oxide Nanoparticles and their characterizations Stud ies,” Nanoscience and Nanotechnology, vol. 2, no. 5, pp. 134– 138, 2012.
M. Arakha, S. Pal, D. Samantarrai et al., “Antimicrobial activity of iron oxide nanoparticle upon modulation of nanoparticle-bacteria interface,” Scientific Reports, vol. 5, no. 1, p. 14813, 2015.
B. S. Inbaraj, T.-Y. Tsai, and B.-H. Chen,“Synthesis, characterization and antibacterial activity of superparamagnetic nanoparticles modified with glycol chitosan,” Science Technology of Advanced Materials, vol. 13, no. 1, article 015002, 2012.
S. Hwang, A. Umar, G. Dar, S. Kim, and R. Badran, “Synthesis and characterization of iron oxide nanoparticles for phenyl hydrazine sensor applications,” Sensor Letters, vol. 12, no. 1, pp. 97–101, 2014.
P. Sharma, R. Kumar, S. Chauhan, D. Singh, and M. S. Chauhan, “Facile growth and characterization of alpha-Fe2O3 nanoparticles for photocatalytic degradation of methyl orange,” Journal of Nanoscience and Nanotechnology, vol. 14, no. 8, pp. 6153–6157, 2014.
P. Saharan, G. R. Chaudhary, S. K. Mehta, and A. Umar, “Removal of water contaminants by iron oxide nanomaterials,” Journal of Nanoscience and Nanotechnology, vol. 14, no. 1, pp. 627–643, 2014.
P. R. Patil and S. S. Joshi, “Synthesis of α-Fe2O3 nanocubes,” Synthesis Reactivity in Inorganic, Metal-Organic, Nano-Metal Chemistry, vol. 37, no. 6, pp. 425–429, 2007.
S. Wang, X. Li, S. Wang, Y. Li, Y. Zhai, Mater. Lett. 62, 3552 (2008)
A. Afkhami, M. Saber-Tehrani, H. Bagheri, J. Hazard. Mater. 181, 836 (2010).
K.M. Batoo, S. Kumar, C.G. Lee, Curr. Appl. Phys. 9, 826 (2009).
Zhang, Z., & Pinnavaia, T. J. (2002). Mesostructured γ-Al2O3 with a lathlike framework morphology. Journal of the American Chemical Society, 124(41), 12294-12301.
A.U. Haq, M. Saeed, M. Usman, M. Yameen, M. Muneer, S. Tubbsum, A comparative sorption study of Cr3+ and Cr6+ using mango peels: kinetic, equilibrium and thermodynamic, Green Processing and Synthesis, 8, 337-347(2019).
X.H. Vu, L.H. Nguyen, H.T. Van, D.V. Nguyen, T.H. Nguyen, Q.T. Nguyen, L. Ha, Adsorption of chromium(VI) onto freshwater snail shell-derived biosorbent from aqueous solutions: Equilibrium, kinetics, and thermodynamics, Journal of Chemistry, Article ID: 3038103, 1-11(2019).
S. Tamjidi, H. Esmaeili, Chemically modified CaO/Fe3O4 nanocomposite by sodium dodecyl sulfate for Cr(III) removal from water, Chemical Engineering and Technology, 42, 607-616(2019).
J. Wang, R. Cao, D. He, A. Saleem, Facile preparation of polyethyleneimine modified activated sludge-based adsorbent for hexavalent chromium removal from aqueous solution, Separation Science and Technology,56,498-506 (2021).
F. Ahmadi, H. Esmaeili, Chemically modified bentonite/Fe3O4 nanocomposite for Pb(II), Cd(II), and Ni(II) removal from synthetic wastewater, Desalination and Water Treatment,110, 154-167(2018).
Mahmoud, M. E., Amira, M. F., Seleim, S. M., & Mohamed, A. K. (2017). Adsorption isotherm models, kinetics study, and thermodynamic parameters of Ni (II) and Zn (II) removal from water using the LbL technique. Journal of Chemical & Engineering Data, 62(2), 839-850.
Darmokoesoemo, H., Kuncoro, E. P., Supriyanto, G., & Manuharab, Y. S. W. (2020). Models, kinetics, and thermodynamics for the adsorption of Pb2+ and Cd2+ metal ions by solid tofu waste immobilized on silica’s SURFAC. Moroccan Journal of Chemistry, 8(1), 8-1.
Darmokoesoemo, H., Kuncoro, E. P., Supriyanto, G., & Manuharab, Y. S. W. (2020). Models, kinetics, and thermodynamics for the adsorption of Pb2+ and Cd2+ metal ions by solid tofu waste immobilized on silica’s SURFAC. Moroccan Journal of Chemistry, 8(1), 8-1.
Darmokoesoemo, H., Kuncoro, E. P., Supriyanto, G., & Manuhara, Y. S. W. (2020). Models, kinetics, and thermodynamics for the adsorption of Pb2+ and Cd2+ metal ions by solid tofu waste immobilized on silica’s SURFACE. Moroccan Journal of Chemistry, 8(S1), 12-23.
Published
How to Cite
Issue
Section
License
Copyright (c) 2022 50sea

This work is licensed under a Creative Commons Attribution 4.0 International License.




















