Effects of Filters in Retinal Disease Detection on Optical Coherence Tomography (OCT) Images Using Machine Learning Classifiers
Keywords:
Random Forest Classifier (RFC), Support Vector Machine (SVM), K-Nearest Neighbor (KNN), Machine Learning, Optical Coherence Tomography (OCT), Diabetic Macular Edema (DME), Choroidal Neovascularization (CNV), DRUSEN, NORMAL., Diabetic Retinopathy (DR), Age Related Macular Degeneration (AMD), Histogram of Oriented Gradients (HOG), Local Binary Patterns (LBP), Features from Opponent Space for Filtering (FOSF)Abstract
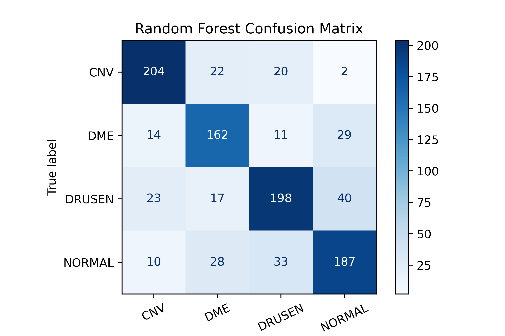
Optical Coherence Tomography (OCT) is an essential, non-invasive imaging technique for producing high-resolution images of the retina, crucial in diagnosing and monitoring retinal conditions such as DME, CNV, and DRUSEN. Despite its importance, there is a pressing need to enhance the early detection and treatment of these common eye diseases. While deep learning methods have shown higher accuracy in classifying OCT images, the potential for machine learning approaches, particularly in terms of data size and computational efficiency, remains underexplored. This study generates models for detecting retinal disease on a publicly available dataset of retinal OCT images using machine learning classifiers with the help of image feature extractions. It classifies the given retinal OCT images as DME, CNV, DRUSEN, and NORMAL. Firstly, it extracts image features using appropriate methods and then it is trained, after training it passes through machine learning classifiers to classify the given input images, and then it is tested to get a better accuracy performance. The above steps are iterated by varying over the pre-processing techniques in which we first resize the image into 100 x 100 after resizing, we remove the noise by using Gaussian Blur and then normalize the image. We systematically benchmark its performance against established built-in methods, such as HOG, LBP, and FOSF. This comparative analysis serves to assess the efficacy of finding the best approach in relation to these widely recognized methods. The proposed experiments based on these approaches reveal that the use of HOG on this dataset outperforms with SVM classifier with a maximum accuracy of 78.8%.
References
J. Jiang et al., “Ultrahigh speed Spectral / Fourier domain OCT ophthalmic imaging at 70,000 to 312,500 axial scans per second,” Opt. Express, Vol. 16, Issue 19, pp. 15149-15169, vol. 16, no. 19, pp. 15149–15169, Sep. 2008, doi: 10.1364/OE.16.015149.
M. E. J. van Velthoven, D. J. Faber, F. D. Verbraak, T. G. van Leeuwen, and M. D. de Smet, “Recent developments in optical coherence tomography for imaging the retina,” Prog. Retin. Eye Res., vol. 26, no. 1, pp. 57–77, Jan. 2007, doi: 10.1016/J.PRETEYERES.2006.10.002.
S. A. Boppart, G. J. Tearney, B. E. Bouma, J. F. Southern, M. E. Brezinski, and J. G. Fujimoto, “Noninvasive assessment of the developing Xenopus cardiovascular system using optical coherence tomography,” Proc. Natl. Acad. Sci. U. S. A., vol. 94, no. 9, pp. 4256–4261, Apr. 1997, doi: 10.1073/PNAS.94.9.4256/ASSET/4849CBA2-F776-442F-86D0-A06D9DB929CE/ASSETS/GRAPHIC/PQ0970492007.JPEG.
M. J. Suter et al., “Intravascular Optical Imaging Technology for Investigating the Coronary Artery,” JACC Cardiovasc. Imaging, vol. 4, no. 9, pp. 1022–1039, Sep. 2011, doi: 10.1016/J.JCMG.2011.03.020.
J. F. Southern et al., “Scanning single-mode fiber optic catheter–endoscope for optical coherence tomography,” Opt. Lett. Vol. 21, Issue 7, pp. 543-545, vol. 21, no. 7, pp. 543–545, Apr. 1996, doi: 10.1364/OL.21.000543.
G. J. Tearney et al., “In Vivo Endoscopic Optical Biopsy with Optical Coherence Tomography,” Science (80-. )., vol. 276, no. 5321, pp. 2037–2039, Jun. 1997, doi: 10.1126/SCIENCE.276.5321.2037.
T. Gambichler, G. Moussa, M. Sand, D. Sand, P. Altmeyer, and K. Hoffmann, “Applications of optical coherence tomography in dermatology,” J. Dermatol. Sci., vol. 40, no. 2, pp. 85–94, Nov. 2005, doi: 10.1016/j.jdermsci.2005.07.006.
J. M. Schmitt, M. J. Yadlowsky, and R. F. Bonner, “Subsurface Imaging of Living Skin with Optical Coherence Microscopy,” Dermatology, vol. 191, no. 2, pp. 93–98, Feb. 1995, doi: 10.1159/000246523.
“Non-invasive ophthalmic imaging of adult zebrafish eye using optical coherence tomography.” Accessed: Feb. 13, 2024. [Online]. Available: https://core.ac.uk/download/pdf/291515351.pdf
J. S. Schuman, L. Kagemann, H. Ishikawa, and G. Wollstein, “Spectral-Domain Optical Coherence Tomography as a Noninvasive Method to Assess Damaged and Regenerating Adult Zebrafish Retinas,” Invest. Ophthalmol. Vis. Sci., vol. 53, no. 11, pp. 7315–7315, Oct. 2012, doi: 10.1167/IOVS.12-10925.
“Optical coherence tomography for high-resolution imaging of mouse development in utero.” Accessed: Feb. 13, 2024. [Online]. Available: https://www.spiedigitallibrary.org/journals/journal-of-biomedical-optics/volume-16/issue-04/046004/Optical-coherence-tomography-for-high-resolution-imaging-of-mouse-development/10.1117/1.3560300.full#_=_
C. A. Stewart, I. V. Larina, J. C. Burton, S. Wang, and R. R. Behringer, “High-resolution three-dimensional in vivo imaging of mouse oviduct using optical coherence tomography,” Biomed. Opt. Express, Vol. 6, Issue 7, pp. 2713-2723, vol. 6, no. 7, pp. 2713–2723, Jul. 2015, doi: 10.1364/BOE.6.002713.
A. Alex et al., “A Circadian Clock Gene, Cry, Affects Heart Morphogenesis and Function in Drosophila as Revealed by Optical Coherence Microscopy,” PLoS One, vol. 10, no. 9, p. e0137236, Sep. 2015, doi: 10.1371/JOURNAL.PONE.0137236.
M. Watanabe et al., “Ultrahigh-speed optical coherence tomography imaging and visualization of the embryonic avian heart using a buffered Fourier Domain Mode Locked laser,” Opt. Express, Vol. 15, Issue 10, pp. 6251-6267, vol. 15, no. 10, pp. 6251–6267, May 2007, doi: 10.1364/OE.15.006251.
F. Shi et al., “Automated 3-D retinal layer segmentation of macular optical coherence tomography images with serous pigment epithelial detachments,” IEEE Trans. Med. Imaging, vol. 34, no. 2, pp. 441–452, Feb. 2015, doi: 10.1109/TMI.2014.2359980.
J. Sugmk, S. Kiattisin, and A. Leelasantitham, “Automated classification between age-related macular degeneration and Diabetic macular edema in OCT image using image segmentation,” BMEiCON 2014 - 7th Biomed. Eng. Int. Conf., Jan. 2014, doi: 10.1109/BMEICON.2014.7017441.
A. Lang, A. Carass, B. M. Jedynak, S. D. Solomon, P. A. Calabresi, and J. L. Prince, “Intensity inhomogeneity correction of macular OCT using N3 and retinal flatspace,” Proc. - Int. Symp. Biomed. Imaging, vol. 2016-June, pp. 197–200, Jun. 2016, doi: 10.1109/ISBI.2016.7493243.
M. Adhi et al., “Choroidal analysis in healthy eyes using swept-source optical coherence tomography compared to spectral domain optical coherence tomography,” Am. J. Ophthalmol., vol. 157, no. 6, pp. 1272-1281.e1, Jun. 2014, doi: 10.1016/j.ajo.2014.02.034.
M. A. Hussain et al., “Classification of healthy and diseased retina using SD-OCT imaging and Random Forest algorithm,” PLoS One, vol. 13, no. 6, p. e0198281, Jun. 2018, doi: 10.1371/JOURNAL.PONE.0198281.
M. Wojtkowski et al., “Ophthalmic imaging by spectral optical coherence tomography,” Am. J. Ophthalmol., vol. 138, no. 3, pp. 412–419, Sep. 2004, doi: 10.1016/j.ajo.2004.04.049.
D. S. Ting, L. R. Pasquale, L. Peng, J. P. Campbell, A. Y. Lee, R. Raman, G. S. Tan, L. Schmetterer, P. A. Keane, and T. Y. Wong, “Artificial intelligence and deep learning in ophthalmology,” British Journal of Ophthalmology, vol. 103, pp. 167–175, 2019.
Schmidt-Erfurth, et al, “Unsupervised identification of disease marker candidates in retinal oct imaging data,” IEEE transactions on medical imaging, vol. 38, pp. 1037–1047, 2019.
Lee C.S., Baughman D.M., Lee A.Y. Deep Learning Is Effective for Classifying Normal versus Age-Related Macular Degeneration OCT Images. Ophthalmol. Retin. 2017;1:322–327. doi: 10.1016/j.oret.2016.12.009.
Kermany DS, Goldbaum M, Cai W, Lewis MA. Identifying Medical Diagnoses and Treatable Diseases by Image-Based Deep Learning Resource Identifying Medical Diagnoses and Treatable Diseases by Image-Based Deep Learning. Cell. 2018;172:1122–31.e9. https://doi.org/10.1016/j.cell.2018.02.010.
Huang L., He X., Fang L., Rabbani H., Chen X. Automatic Classification of Retinal Optical Coherence Tomography Images With Layer Guided Convolutional Neural Network. IEEE Signal Process. Lett. 2019;26:1026–1030. doi: 10.1109/LSP.2019.2917779.
Chowdhary C.L., Acharjya D. Clustering Algorithm in Possibilistic Exponential Fuzzy C-Mean Segmenting Medical Images. J. Biomimetics Biomater. Biomed. Eng. 2017;30:12–23. doi: 10.4028/www.scientific.net/JBBBE.30.12.
T. Tsuji et al., “Classification of optical coherence tomography images using a capsule network,” BMC Ophthalmol., vol. 20, no. 1, pp. 1–9, Mar. 2020, doi: 10.1186/S12886-020-01382-4/FIGURES/9.
G. Latha and P. Aruna Priya, “Glaucoma Retinal Image Detection and Classification using Machine Learning Algorithms,” J. Phys. Conf. Ser., vol. 2335, no. 1, p. 012025, Sep. 2022, doi: 10.1088/1742-6596/2335/1/012025.
Y. Zhou, “Automated Identification of Diabetic Retinopathy Using Deep Learning,” 2021.
Jian Li, “Automated Detection and Classification of Diabetic Retinopathy Using Deep Learning Based on EfficientNet,” 2020.
H. Fu, “Automated diagnosis of diabetic retinopathy using deep learning”, 2018
S. W. Ting et al., “Artificial intelligence and deep learning in ophthalmology,” Br. J. Ophthalmol., vol. 103, no. 2, pp. 167–175, Feb. 2019, doi: 10.1136/BJOPHTHALMOL-2018-313173.
R. Gargeya and T. Leng, “Automated Identification of Diabetic Retinopathy Using Deep Learning,” Ophthalmology, vol. 124, no. 7, pp. 962–969, Jul. 2017, doi: 10.1016/J.OPHTHA.2017.02.008.
Hwang D.K., Hsu C.C., Chang K.J., Chao D., Sun C.H., Jheng Y.C., Yarmishyn A.A., Wu J.C., Tsai C.Y., Wang M.L., et al. Artificial intelligence-based decision-making for age-related macular degeneration. Theranostics. 2019;9:232–245. doi: 10.7150/thno.28447.
Tasnim N., Hasan M., Islam I. Comparisonal study of Deep Learning approaches on Retinal OCT Image. arXiv. 20191912.07783
Published
How to Cite
Issue
Section
License
Copyright (c) 2024 50SEA

This work is licensed under a Creative Commons Attribution 4.0 International License.




















