Capturing CO2 and Recovering NH3 by Producing Ammonium Bicarbonate Through Stripping Batch Process at Lab Scale
Keywords:
Ammonium Bicarbonate, Ammonium Hydroxide, Carbon dioxide, Stripping Batch Process, RecoveringAbstract
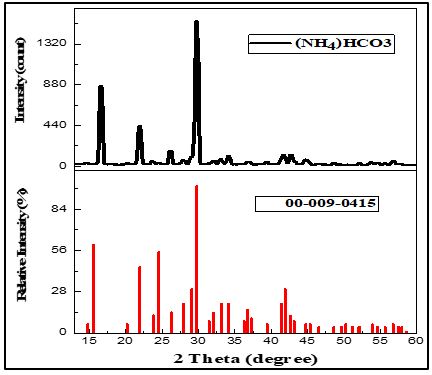
A sustainable Environment is a crucial need for today’s world. To save energy, reduce pollution, and save the economy, researchers are coming up with various sustainable waste management practices to reduce air pollution and water pollution. The production of cement, chemical processing, and power plants are among the industries that release the most CO2. These emissions can be greatly decreased via ammonia-based absorption, which helps to make industrial processes cleaner. One of the methods among all the technologies and solutions is the stripping process where CO2 can be captured by removing ammonia from the water. Not only this but also the chemical is produced NH4HCO3 which can be used in industries or as fertilizer. In this study, A lab-scale stripping process is studied for the recovery of ammonia and capture of CO2 at different experimental conditions which were not studied by other researchers in previous studies. Apart from that, the precipitated product is studied by various characterization techniques including SEM-EDS and XRD. Results show that varying absorption times and flow rates of CO2 and concentrations of solutions affect product quantity. The research concludes the optimum conditions to achieve maximum product i.e., NH4HCO3 was 110 min, 0.5 CO2 gas flow rate, and 15 % NH4OH solution.
References
D. Y. C. Leung, G. Caramanna, and M. M. Maroto-Valer, “An overview of current status of carbon dioxide capture and storage technologies,” Renew. Sustain. Energy Rev., vol. 39, pp. 426–443, Nov. 2014, doi: 10.1016/J.RSER.2014.07.093.
A. C. Yeh and H. Bai, “Comparison of ammonia and monoethanolamine solvents to reduce CO2 greenhouse gas emissions,” Sci. Total Environ., vol. 228, no. 2–3, pp. 121–133, Apr. 1999, doi: 10.1016/S0048-9697(99)00025-X.
H. Bai and A. C. Yeh, “Removal of CO2 Greenhouse Gas by Ammonia Scrubbing,” Ind. Eng. Chem. Res., vol. 36, no. 6, pp. 2490–2493, 1997, doi: 10.1021/IE960748J.
N. Dave, T. Do, G. Puxty, R. Rowland, P. H. M. Feron, and M. I. Attalla, “CO2 capture by aqueous amines and aqueous ammonia–A Comparison,” Energy Procedia, vol. 1, no. 1, pp. 949–954, Feb. 2009, doi: 10.1016/J.EGYPRO.2009.01.126.
K. Han, C. K. Ahn, and M. S. Lee, “Performance of an ammonia-based CO2 capture pilot facility in iron and steel industry,” Int. J. Greenh. Gas Control, vol. 27, pp. 239–246, Aug. 2014, doi: 10.1016/J.IJGGC.2014.05.014.
P. C. Chen and S. C. Yu, “CO2 Capture and Crystallization of Ammonia Bicarbonate in a Lab-Scale Scrubber,” Cryst. 2018, Vol. 8, Page 39, vol. 8, no. 1, p. 39, Jan. 2018, doi: 10.3390/CRYST8010039.
M. Brondi, M. Eisa, R. Bortoletto-Santos, D. Drapanauskaite, T. Reddington, C. Williams, et al., "Recovering, Stabilizing, and Reusing Nitrogen and Carbon from Nutrient-Containing Liquid Waste as Ammonium Carbonate Fertilizer," Agriculture, vol. 13, p. 909, 2023.
Q. Zhuang, B. Clements, and Y. Li, “From ammonium bicarbonate fertilizer production process to power plant CO2 capture,” Int. J. Greenh. Gas Control, vol. 10, pp. 56–63, Sep. 2012, doi: 10.1016/J.IJGGC.2012.05.019.
“Anonymous (2000) Fertilizers and Their Use. FAO, International Industry Association, Rome, 35-38. - References - Scientific Research Publishing.” Accessed: Jun. 11, 2024. [Online]. Available: https://www.scirp.org/reference/referencespapers?referenceid=1230250
M. M. A. Al-Shammari, “The Enhancement of the Ammonium Bicarbonate Synthesis Using Non-Thermal Plasma,” 2020.
S. Bavarella, A. Brookes, A. Moore, P. Vale, G. Di Profio, E. Curcio, et al., "Chemically reactive membrane crystallisation reactor for CO2–NH3 absorption and ammonium bicarbonate crystallisation: Kinetics of heterogeneous crystal growth," Journal of Membrane Science, vol. 599, p. 117682, 2020.
A. S. Hamouda, M. S. Eldien, and M. F. Abadir, “Carbon dioxide capture by ammonium hydroxide solution and its possible application in cement industry,” Ain Shams Eng. J., vol. 11, no. 4, pp. 1061–1067, Dec. 2020, doi: 10.1016/J.ASEJ.2020.01.001.
“511, C.N. National Institute of Standards and Technology U.S. Department of Commerce”, [Online]. Available: https://webbook.nist.gov/cgi/cbook.cgi?ID=C1066337&Mask=80
P. C. Chen et al., “Interpretation of gas–liquid reactive crystallization data using a size-independent agglomeration model,” J. Cryst. Growth, vol. 257, no. 3–4, pp. 333–343, Oct. 2003, doi: 10.1016/S0022-0248(03)01425-8.
Downloads
Published
How to Cite
Issue
Section
License
Copyright (c) 2024 50SEA

This work is licensed under a Creative Commons Attribution 4.0 International License.




















