Osteochondroma Identification Through Transfer Learning and Convolutional Neural Networks
Keywords:
Osteochondroma, Deep Learning, Convolutional Neural Networks, Transfer Learning, Medical Imaging, Image ClassificationAbstract
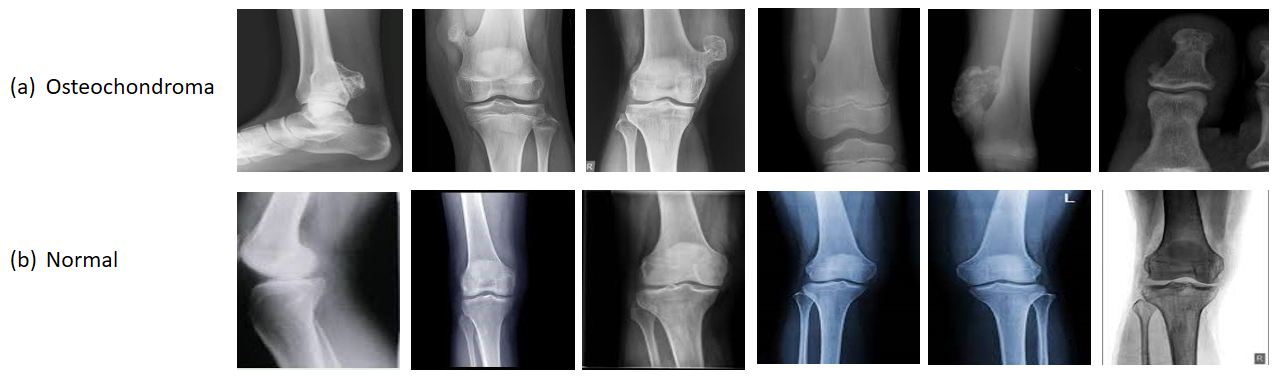
Accurate and timely diagnosis of musculoskeletal conditions like osteochondroma is pivotal in ensuring effective treatment and improved patient outcomes. However, traditional diagnostic methods relying on manual interpretation of medical images can be susceptible to human errors, potentially leading to misdiagnosis or delayed detection. Previous studies have explored Deep Learning (DL) techniques for automated disease detection, but they often face challenges such as limited dataset availability and generalization capabilities across diverse imaging modalities. This research addresses these gaps by proposing a robust Convolutional Neural Network (CNN) framework for osteochondroma identification, leveraging transfer learning and data augmentation techniques. The ResNet-50 architecture, pre-trained on a large dataset, is fine-tuned with dense layers and an output layer for binary classification. Extensive data pre-processing and offline augmentation strategies enhance model performance and generalizability. The proposed model achieves an impressive 97.67% accuracy on the test dataset, demonstrating its effectiveness in distinguishing between normal and osteochondroma cases. Furthermore, its generalizability is validated by training and testing on the publicly available Potato Leaf Disease dataset, showcasing consistent performance in multi-class classification scenarios. While the model exhibits promising results, future work could explore integrating more extensive and diverse datasets and investigating advanced architectures for improved accuracy and computational efficiency. The implications of this research extend to empowering medical practitioners with accurate and swift osteochondroma diagnostics, ultimately contributing to enhanced patient care in orthopaedics.
References
K. Tepelenis et al., “Osteochondromas: An Updated Review of Epidemiology, Pathogenesis, Clinical Presentation, Radiological Features and Treatment Options,” In Vivo (Brooklyn)., vol. 35, no. 2, pp. 681–691, Mar. 2021, doi: 10.21873/INVIVO.12308.
M. D. Murphey, J. J. Choi, M. J. Kransdorf, D. J. Flemming, and F. H. Gannon, “Imaging of Osteochondroma: Variants and Complications with Radiologic-Pathologic Correlation1,” https://doi.org/10.1148/radiographics.20.5.g00se171407, vol. 20, no. 5, pp. 1407–1434, Sep. 2000, doi: 10.1148/RADIOGRAPHICS.20.5.G00SE171407.
S. M. M. Doctors Marco Cañete P, Elena Fontoira M, Begoña Gutiérrez San José, “Osteochondroma: radiological diagnosis, complications and variants,” 2013, [Online]. Available: https://www.webcir.org/revistavirtual/articulos/septiembre13/chile/ch_ing.pdf
E. Farooq, M. A. Nawaz Ui Ghani, Z. Naseer, and S. Iqbal, “Privacy Policies’ Readability Analysis of Contemporary Free Healthcare Apps,” 2020 14th Int. Conf. Open Source Syst. Technol. ICOSST 2020 - Proc., Dec. 2020, doi: 10.1109/ICOSST51357.2020.9332991.
T. Zhao and H. Zhao, “Computed Tomographic Image Processing and Reconstruction in the Diagnosis of Rare Osteochondroma,” Comput. Math. Methods Med., vol. 2021, 2021, doi: 10.1155/2021/2827556.
E. Farooq and A. Borghesi, “A Federated Learning Approach for Anomaly Detection in High Performance Computing,” Proc. - Int. Conf. Tools with Artif. Intell. ICTAI, pp. 496–500, 2023, doi: 10.1109/ICTAI59109.2023.00079.
L. Pinto-Coelho, “How Artificial Intelligence Is Shaping Medical Imaging Technology: A Survey of Innovations and Applications,” Bioeng. 2023, Vol. 10, Page 1435, vol. 10, no. 12, p. 1435, Dec. 2023, doi: 10.3390/BIOENGINEERING10121435.
F. M. Javed Mehedi Shamrat et al., “LungNet22: A Fine-Tuned Model for Multiclass Classification and Prediction of Lung Disease Using X-ray Images,” J. Pers. Med., vol. 12, no. 5, p. 680, May 2022, doi: 10.3390/JPM12050680/S1.
N. Ullah, M. S. Khan, J. A. Khan, A. Choi, and M. S. Anwar, “A Robust End-to-End Deep Learning-Based Approach for Effective and Reliable BTD Using MR Images,” Sensors 2022, Vol. 22, Page 7575, vol. 22, no. 19, p. 7575, Oct. 2022, doi: 10.3390/S22197575.
M. Thilagaraj, N. Arunkumar, and P. Govindan, “Classification of Breast Cancer Images by Implementing Improved DCNN with Artificial Fish School Model,” Comput. Intell. Neurosci., vol. 2022, 2022, doi: 10.1155/2022/6785707.
H. Malik et al., “A Novel Fusion Model of Hand-Crafted Features With Deep Convolutional Neural Networks for Classification of Several Chest Diseases Using X-Ray Images,” IEEE Access, vol. 11, pp. 39243–39268, 2023, doi: 10.1109/ACCESS.2023.3267492.
U. C. Aytaç, A. Güneş, and N. Ajlouni, “A novel adaptive momentum method for medical image classification using convolutional neural network,” BMC Med. Imaging, vol. 22, no. 1, pp. 1–12, Dec. 2022, doi: 10.1186/S12880-022-00755-Z/FIGURES/5.
F. R. Eweje et al., “Deep Learning for Classification of Bone Lesions on Routine MRI,” EBioMedicine, vol. 68, p. 103402, Jun. 2021, doi: 10.1016/j.ebiom.2021.103402.
K. Sampath, S. Rajagopal, and A. Chintanpalli, “A comparative analysis of CNN-based deep learning architectures for early diagnosis of bone cancer using CT images,” Sci. Reports 2024 141, vol. 14, no. 1, pp. 1–9, Jan. 2024, doi: 10.1038/s41598-024-52719-8.
W. Chen et al., “A fusion of VGG-16 and ViT models for improving bone tumor classification in computed tomography,” J. Bone Oncol., vol. 43, p. 100508, Dec. 2023, doi: 10.1016/J.JBO.2023.100508.
R. Anand, S. V. Lakshmi, D. Pandey, and B. K. Pandey, “An enhanced ResNet-50 deep learning model for arrhythmia detection using electrocardiogram biomedical indicators,” Evol. Syst., vol. 15, no. 1, pp. 83–97, Feb. 2024, doi: 10.1007/S12530-023-09559-0/METRICS.
Downloads
Published
How to Cite
Issue
Section
License
Copyright (c) 2024 50SEA

This work is licensed under a Creative Commons Attribution 4.0 International License.




















