Advanced Blast Algorithm for Molecular Identification, Biodegradation and Decolorization of Synthetic Melanoidins Using Fungal Species Isolated from Soil and Spent Wash
Keywords:
Synthetic Melanoidins, Biodegradation and Bioremediation, Indigenous Fungal Species, Spent Wash, Molecular IdentificationAbstract
Introduction/Importance of Study: Distillery spent wash contains a high organic load as Melanoidins. It is generated due to the Millard reaction, which produces sugar and amino acids, leading to extensive water and soil pollution. Anaerobic digestion removes 60-70% COD and color, so post treatment is required for degradation by using fungal species as biological process.
Objectives and Novelty statement for this study: The study aims to isolate and identify fungal species for the degradation of synthetic melanoidins from spent wash using a cost-effective, low-toxicity, and environmentally friendly fungal-based biological process.
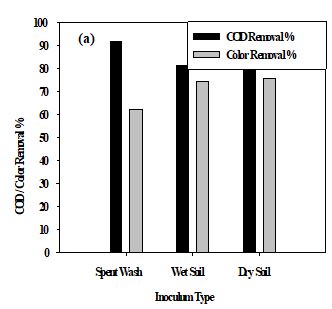
Material and Method: Three mixed fungal culture inoculums (spent wash, wet, and dry soil) and seven isolated fungal strains were examined on solid media that degraded and decolorized melanoidins at controlled pH 5.5, 25oC, 160 rpm for 3-5 days.
Result and Discussion: The results showed that mixed culture of spent wash removed the highest COD 91.8 %, color removal was 75.7 %, F-S6 isolate identified as Penicillium showed maximum soluble COD removal was 96.7 %, and F-S5 isolate identified as Syncephalastrum showed a maximum color removal was 98.8 %.
Concluding Remarks: It was concluded that the microbial process using fungal species was successfully applied to enhance degradation and decolorization to remove melanoidins. Furthermore, Gompertz Modeling was done to check the fitting of the curve at 680 nm Optical Density (OD) analysis for seven fungal strains with the following five factors significantly estimating maximum specific growth rate µM, Asymptote A, coefficient of determination R2, lag time λ, and goodness of fit.
References
Chavan, K.e.a., Microbial degradation of melanoidins in distillery spent wash by an indiginious isolate. 2006.
Arun Kumar, S.A.e.a., Comparitive Study on Potentiality of Bacteria and Fungi in Bioremediation and Decolorization of Molasses Spent Wash. 2008.
Chandra, R. and V. Kumar, Detection of Bacillus and Stenotrophomonas species growing in an organic acid and endocrine-disrupting chemical-rich environment of distillery spent wash and its phytotoxicity. Environmental Monitoring and Assessment, 2016. 189(1): p. 26.
Khandekar, Y.S. and N.P. Shinkar, DISTILLERY SPENT WASH BIOLOGICAL TREATMENT TECHNIQUES: A REVIEW. 2020.
Kulkarni, S.P., V.R. Kakde, and K.A. Mane, Treatments to Distillery Spent wash by Electro coagulation [EC] and Adsorption: A Review. International Journal of Engineering Research and General Science, 2016. 4(3).
Raghukumar, C., et al., Simultaneous detoxification and decolorization of molasses spent wash by the immobilized white-rot fungus Flavodon flavus isolated from a marine habitat. Enzyme and Microbial Technology, 2004. 35(2-3): p. 197-202.
Satyawali, Y. and M. Balakrishnan, Wastewater treatment in molasses-based alcohol distilleries for COD and color removal: A review. Journal of Environmental Management, 2008. 86(3): p. 481-497.
Sirianuntapiboon, S., P. Zohsalam, and S. Ohmomo, Decolorization of molasses wastewater by Citeromyces sp. WR-43-6. Process biochemistry, 2004. 39(8): p. 917-924.
Wagh, M.P. and P.D. Nemade, Treatment processes and technologies for decolourization and COD removal of distillery spent wash: a review. Int. J. Innov. Res. Adv. Engine, 2015. 7(2): p. 30-40.
Langner, E. and W. Rzeski, Biological Properties of Melanoidins: A Review. International Journal of Food Properties, 2014. 17(2): p. 344-353.
Munde, S. and T. Bhattacharjee, Distillery Spent Wash Treatment: Model Degradationof Synthetic Melanoidin by In-Situ Chemical Oxidation. International Journal of Chemical and Physical Sciences, 2015. 4: p. 128-136.
Fitzgibbon, F.J., et al., Biological treatment of distillery waste for pollution‐remediation. Journal of Basic Microbiology, 1995. 35(5): p. 293-301.
Singh, N. and A. Gangotra, Biodegradation of Melanoidin from Distillery Effluent: Role of Microbes and Their Potential Enzymes. 2013. p. 71-104.
Papetti, A., et al., Isolation of an in Vitro and ex Vivo Antiradical Melanoidin from Roasted Barley. Journal of Agricultural and Food Chemistry, 2006. 54(4): p. 1209-1216.
Rufián-Henares, J.A. and S. Pastoriza, Maillard Reaction, in Encyclopedia of Food and Health, B. Caballero, P.M. Finglas, and F. Toldrá, Editors. 2016, Academic Press: Oxford. p. 593-600.
Rufián-Henares, J.A. and F.J. Morales, Functional properties of melanoidins: In vitro antioxidant, antimicrobial and antihypertensive activities. Food Research International, 2007. 40(8): p. 995-1002.
Manisankar, P., S. Viswanathan, and C. Rani, Electrochemical treatment of distillery effluent using catalytic anodes. Green chemistry, 2003. 5(2): p. 270-274.
Nataraj, S.K., K.M. Hosamani, and T.M. Aminabhavi, Distillery wastewater treatment by the membrane-based nanofiltration and reverse osmosis processes. Water Research, 2006. 40(12): p. 2349-2356.
Pala, A. and G. Erden, Decolorization of a baker's yeast industry effluent by Fenton oxidation. Journal of hazardous materials, 2005. 127(1-3): p. 141-148.
Chaturvedi, S., R. Chandra, and V. Rai, Isolation and characterization of Phragmites australis (L.) rhizosphere bacteria from contaminated site for bioremediation of colored distillery effluent. Ecological Engineering, 2006. 27(3): p. 202-207.
Sirianuntapiboon, S., P. Phothilangka, and S. Ohmomo, Decolorization of molasses wastewater by a strain No.BP103 of acetogenic bacteria. Bioresource Technology, 2004. 92(1): p. 31-39.
Pillai H P, J., et al., Microbial decolourization and bioremediation of molasses waste water. Journal of Natural Sciences, 2012. 2: p. 914-920.
Chandra, R., P.K. Pandey, and A. Srivastava, Comparative toxicological evaluation of untreated and treated tannery effluent with Nostoc Muscorum L.(Algal assay) and microtox bioassay. Environmental monitoring and assessment, 2004. 95(1): p. 287-294.
Fahy, V., et al., Decolourisation of molasses spent wash by Phanerochaete chrysosporium. Biotechnology Letters, 1997. 19(1): p. 97-99.
Miyata, N., et al., Microbial decolorization of melanoidin-containing wastewaters: combined use of activated sludge and the fungus Coriolus hirsutus. Journal of bioscience and bioengineering, 2000. 89(2): p. 145-150.
Raghukumar, C. and G. Rivonkar, Decolorization of molasses spent wash by the white-rot fungus Flavodon flavus, isolated from a marine habitat. Applied Microbiology and Biotechnology, 2001. 55: p. 510-514.
Apha, A., Standard methods for the examination of water and wastewater: Apha Washington. 1985.
Chandra, R., V. Kumar, and S. Tripathi, Evaluation of molasses-melanoidin decolourisation by potential bacterial consortium discharged in distillery effluent. 3 Biotech, 2018. 8(4): p. 1-16.
Federation, W.E. and A.P.H. Association, Standard methods for the examination of water and wastewater. American Public Health Association (APHA): Washington, DC, USA, 2005.
Lorenz, K., SCREENING AND IDENTIFICATION OF BACTERIA AND OPTIMIZATION OF PROCESS PARAMETERS FOR DECOLOURIZATION AND DETOXIFICATION OF DISTILLERY MILL EFFLUENT.
Bernardo, E., R. Egashira, and J. Kawasaki, Decolorization of molasses' wastewater using activated carbon prepared from cane bagasse. Carbon, 1997. 35(9): p. 1217-1221.
Kumar, P. and R. Chandra, Decolourisation and detoxification of synthetic molasses melanoidins by individual and mixed cultures of Bacillus spp. Bioresour Technol, 2006. 97(16): p. 2096-102.
Pal, S. and Y. Vimala, Bioremediation and decolorization of Distillery effluent by novel Microbial Consortium. EUROPEAN JOURNAL OF EXPERIMENTAL BIOLOGY, 2012. 28763: p. 496.
Sirianuntapiboon, S., et al., Screening of filamentous fungi having the ability to decolorize molasses pigments. Agricultural and biological chemistry, 1988. 52(2): p. 387-392.
Thakur, I.S., Screening and identification of microbial strains for removal of colour and adsorbable organic halogens in pulp and paper mill effluent. Process Biochemistry, 2004. 39(11): p. 1693-1699.
Dahiya, J., D. Singh, and P. Nigam, Decolourisation of synthetic and spentwash melanoidins using the white-rot fungus Phanerochaete chrysosporium JAG-40. Bioresource Technology, 2001. 78(1): p. 95-98.
Sirianuntapiboon, S., K. Chairattanawan, and S. Ohmomo, Removal of colored substances from molasses waste water by biological treatment systems combined with chemical treatment. JAPAN AGRICULTURAL RESEARCH QUARTERLY, 1998. 32: p. 211-216.
Rane, M.D., P. Rathi, and R.R. Tambe, Microbial decolorization and bioremediation of melanoidin containing molasses spent wash. 2018.
Onyango, M.S., et al., Simultaneous adsorption and biodegradation of synthetic melanoidin. African Journal of Biotechnology, 2012. 11(22): p. 6083-6090.
Bergey, D.H., et al., Manual of determinative bacteriology. Manual of determinative bacteriology. Fifth Edn., 1939.
Brown, A. and H. Smith, Benson’s Microbiological Applications, Laboratory Manual in General Microbiology, Short Version. 2014: McGraw-Hill Education.
Brown, A.E., Microbiological Applications. McGRAW-Hill Companies, 2005. 35: p. 217-224.
Nishiguchi, M.K., et al., DNA isolation procedures. 2002, Springer.
Reeb, D., et al., Fungi associated with the skin of a southern right whale (Eubalaena australis) from South Africa. Mycology, 2010. 1(3): p. 155-162.
Cuppers, H. and J. Smelt, Time to turbidity measurement as a tool for modeling spoilage by Lactobacillus. Journal of industrial microbiology and biotechnology, 1993. 12(3-5): p. 168-171.
Manyuchi, M., et al., KINETIC MODELLING FOR BIO-METHANE GENERATION DURING ANAEROBIC DIGESTION OF MUNICIPAL SEWAGE SLUDGE UTILIZING ACTI-ZYME (BIO-CATALYST) AS A RESOURCE RECOVERY STRATEGY. Chemical Science, 2015. 5: p. 29-37.
Ramya, M., et al., Biodecolorization and biodegradation of Reactive Blue by Aspergillus sp. African Journal of Biotechnology, 2007. 6(12).
Wedzicha, B.L. and M.T. Kaputo, Melanoidins from glucose and glycine: composition, characteristics and reactivity towards sulphite ion. Food Chemistry, 1992. 43(5): p. 359-367.
Fahy, V., et al., Decolourisation of molasses spent wash by Phanerochaete chrysosporium. Biotechnology Letters, 1997. 19(1): p. 97-99.
Ojijo, V.O., et al., Decolourization of melanoidin containing wastewater using South African coal fly ash. International Journal of Civil and Environmental Engineering, 2010. 2(1): p. 17-23.
Royer, G., et al., Continuous decolorization of bleached kraft effluents by Coriolus versicolor in the form of pellets. Journal of industrial microbiology, 1991. 7(4): p. 269-277.
Al-Keridis, L.A., Application of Penicillium sp as Entomopathogenic Fungi to Control the Red Rust Beetle Tribolium castaneum (Hbst.)(Coleoptera: Tenebrionidae). Biosciences Biotechnology Research Asia, 2016. 12(Spl. Edn. 2): p. 07-12.
Pant, D. and A. Adholeya, Biological approaches for treatment of distillery wastewater: a review. Bioresource technology, 2007. 98(12): p. 2321-2334.
Sankaran, K., et al., DEPHY project: distillery wastewater treatment through anaerobic digestion and phycoremediation—a green industrial approach. Renewable and Sustainable Energy Reviews, 2014. 37: p. 634-643.
Maurice, S.O., Simultaneous adsorption and biodegradation of synthetic melanoidin. African Journal of Biotechnology, 2012. 11(22).
Mohana, S., C. Desai, and D. Madamwar, Biodegradation and decolourization of anaerobically treated distillery spent wash by a novel bacterial consortium. Bioresource Technology, 2007. 98(2): p. 333-339.
Ravikumar, A., et al., Piezoelectric nanogenerator induced work function on a metal phenolic coordination framework from copper oxide nanospheres for efficient biomechanical energy harvesting and physiological monitoring. Journal of Materials Chemistry C, 2022. 10(43): p. 16492-16505.
Baty, F., J.-P. Flandrois, and M.L. Delignette-Muller, Modeling the lag time of Listeria Monocytogenes from viable count enumeration and optical density data. Applied and environmental microbiology, 2002. 68(12): p. 5816-5825.
Mahgoub, S., C. Tsioptsias, and P. Samaras, Biodegradation and decolorization of melanoidin solutions by manganese peroxidase yeasts. Water Science and Technology, 2016. 73(10): p. 2436-2445.
Ohmomo, S., et al., Decolorization of molasses waste water by a thermophilic strain, Aspergillus fumigatus G-2-6. Agricultural and biological chemistry, 1987. 51(12): p. 3339-3346.
McKellar, R.C. and K. Knight, A combined discrete–continuous model describing the lag phase of Listeria monocytogenes. International journal of food microbiology, 2000. 54(3): p. 171-180.
Mytilinaios, I., et al., Growth curve prediction from optical density data. International journal of food microbiology, 2012. 154(3): p. 169-176.
Royle, J.A., et al., Chapter 3 - GLMs and Bayesian Analysis, in Spatial Capture-recapture, J.A. Royle, et al., Editors. 2014, Academic Press: Boston. p. 47-85.
Tiwari, S., et al., A novel thermotolerant Pediococcus acidilactici B-25 strain for color, COD, and BOD reduction of distillery effluent for end use applications. Environ Sci Pollut Res Int, 2013. 20(6): p. 4046-58.
Raghukumar, C., et al., Simultaneous detoxification and decolorization of molasses spent wash by the immobilized white-rot fungus Flavodon flavus isolated from a marine habitat. Enzyme and Microbial Technology, 2004. 35(2-3): p. 197-202.
Kaushik, G. and I. Thakur, Isolation and characterization of distillery spent wash color reducing bacteria and process optimization by Taguchi approach. International Bioodeterioration and Biodegradation, 2009. 63.
Chavan, M., et al., Microbial degradation of melanoidins in distillery spent wash by an indigenous isolate. Indian Journal of biotechnology, 2006. 5: p. 416-421.
Downloads
Published
How to Cite
Issue
Section
License
Copyright (c) 2024 50SEA

This work is licensed under a Creative Commons Attribution 4.0 International License.




















